Jackson, Stephen Matthew (1998) Foraging Ecology, Behaviour and Management of the Mahogany Glider Petaurus Gracilis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Husbandry Guidelines for Common Ringtail Possums, Pseudocheirus Peregrinus Mammalia: Pseudocheiridae
32325/01 Casey Poolman E0190918 Husbandry guidelines for Common Ringtail Possums, Pseudocheirus peregrinus Mammalia: Pseudocheiridae Ault Ringtail Possum Image: Casey Poolman Author: Casey Poolman Date of preparation: 7/11/2017 Open Colleges, Course name and number: ACM30310 Certificate III in Captive Animals Trainer: Chris Hosking Husbandry guidelines for Pseudocheirus peregrinus 1 32325/01 Casey Poolman E0190918 Author contact details [email protected] Disclaimer Please note that these husbandry guidelines are student material, created as part of student assessment for Open Colleges ACM30310 Certificate III in Captive Animals. While care has been taken by students to compile accurate and complete material at the time of creation, all information contained should be interpreted with care. No responsibility is assumed for any loss or damage resulting from using these guidelines. Husbandry guidelines are evolving documents that need to be updated regularly as more information becomes available and industry knowledge about animal welfare and care is extended. Husbandry guidelines for Pseudocheirus peregrinus 2 32325/01 Casey Poolman E0190918 Workplace Health and Safety risks warning Ringtail Possums are not an aggressive possum and will mostly try to freeze or hide when handled, however they can and do bite, which can be deep and penetrating. When handling possums always be careful not to get bitten, do not put your hands around its mouth. You should always use two hands and be firm but gentle. Adult Ringtail Possums should be gripped by the back of the neck and around the shoulders with one hand and around the base of the tail with the other. This should allow you to control the animal without hurting it and reduces the risk of you being bitten or scratched. -

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

The Mahogany Glider Critical Habitats That Fall Within the Bounds of State
ISSN 1327-8231 ECONOMICS, ECOLOGY AND THE ENVIRONMENT Working Paper No. 100 An Initial Assessment of Policies for Saving a Rare Australian Glider: Experimental Results, Economics and Ecology by Clem Tisdell, Clevo Wilson and Hemanath Swarna Nantha May 2004 THE UNIVERSITY OF QUEENSLAND ISSN 1327-8231 WORKING PAPERS ON ECONOMICS, ECOLOGY AND THE ENVIRONMENT Working Paper No. 100 An Initial Assessment of Policies for Saving a Rare Australian Glider: Experimental Results, Economics and Ecology1 by Clem Tisdell2, Clevo Wilson3 and Hemanath Swarna Nantha4 May 2004 © All rights reserved 1 Note that this is only a draft document and feedback on it is invited. Research for this project has been supported by an Australian Research Council Discovery Grant. We wish to thank Craig Moseley for helping superbly with the maps and Dr Steven Van Dyck for his excellent lecture about the mahogany glider given to survey participants. There are also many others who have assisted us in this research, too many to list here. We are grateful for their help and particularly to the staff of Fleay’s Wildlife Park for their assistance with our third survey 2 School of Economics, The University of Queensland, Brisbane QLD 4072, Australia Email: [email protected] 3 School of Economics, The University of Queensland, Brisbane QLD 4072, Australia Email: [email protected] 4 School of Economics, The University of Queensland, Brisbane QLD 4072, Australia Email: [email protected] WORKING PAPERS IN THE SERIES, Economics, Ecology and the Environment are published by the School of Economics, University of Queensland, 4072, Australia, as follow up to the Australian Centre for International Agricultural Research Project 40 of which Professor Clem Tisdell was the Project Leader. -

Australia Lacks Stem Succulents but Is It Depauperate in Plants With
Available online at www.sciencedirect.com ScienceDirect Australia lacks stem succulents but is it depauperate in plants with crassulacean acid metabolism (CAM)? 1,2 3 3 Joseph AM Holtum , Lillian P Hancock , Erika J Edwards , 4 5 6 Michael D Crisp , Darren M Crayn , Rowan Sage and 2 Klaus Winter In the flora of Australia, the driest vegetated continent, [1,2,3]. Crassulacean acid metabolism (CAM), a water- crassulacean acid metabolism (CAM), the most water-use use efficient form of photosynthesis typically associated efficient form of photosynthesis, is documented in only 0.6% of with leaf and stem succulence, also appears poorly repre- native species. Most are epiphytes and only seven terrestrial. sented in Australia. If 6% of vascular plants worldwide However, much of Australia is unsurveyed, and carbon isotope exhibit CAM [4], Australia should host 1300 CAM signature, commonly used to assess photosynthetic pathway species [5]. At present CAM has been documented in diversity, does not distinguish between plants with low-levels of only 120 named species (Table 1). Most are epiphytes, a CAM and C3 plants. We provide the first census of CAM for the mere seven are terrestrial. Australian flora and suggest that the real frequency of CAM in the flora is double that currently known, with the number of Ellenberg [2] suggested that rainfall in arid Australia is too terrestrial CAM species probably 10-fold greater. Still unpredictable to support the massive water-storing suc- unresolved is the question why the large stem-succulent life — culent life-form found amongst cacti, agaves and form is absent from the native Australian flora even though euphorbs. -

Gtr Pnw343.Pdf
Abstract Marcot, Bruce G. 1995. Owls of old forests of the world. Gen. Tech. Rep. PNW- GTR-343. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 64 p. A review of literature on habitat associations of owls of the world revealed that about 83 species of owls among 18 genera are known or suspected to be closely asso- ciated with old forests. Old forest is defined as old-growth or undisturbed forests, typically with dense canopies. The 83 owl species include 70 tropical and 13 tem- perate forms. Specific habitat associations have been studied for only 12 species (7 tropical and 5 temperate), whereas about 71 species (63 tropical and 8 temperate) remain mostly unstudied. Some 26 species (31 percent of all owls known or sus- pected to be associated with old forests in the tropics) are entirely or mostly restricted to tropical islands. Threats to old-forest owls, particularly the island forms, include conversion of old upland forests, use of pesticides, loss of riparian gallery forests, and loss of trees with cavities for nests or roosts. Conservation of old-forest owls should include (1) studies and inventories of habitat associations, particularly for little-studied tropical and insular species; (2) protection of specific, existing temperate and tropical old-forest tracts; and (3) studies to determine if reforestation and vege- tation manipulation can restore or maintain habitat conditions. An appendix describes vocalizations of all species of Strix and the related genus Ciccaba. Keywords: Owls, old growth, old-growth forest, late-successional forests, spotted owl, owl calls, owl conservation, tropical forests, literature review. -
Abelmoschus Moschatus Subsp
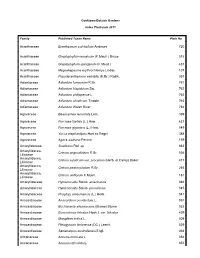
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Coleeae: Crescentieae: Oroxyleae
Gasson & Dobbins - Trees versus lianas in Bignoniaceae 415 Schenck, H. 1893. Beitriige zur Anatomie Takhtajan, A. 1987. Systema Magnoliophy der Lianen. In: A.F.W. Schimper (ed.): torum. Academia Scientiarum U.R.S.S., 1-271. Bot. Mitt. aus den Tropen. Heft Leningrad. 5, Teil2. Gustav Fischer, Jena. Wheeler, E.A., R.G. Pearson, C.A. La Spackman, W. & B.G.L. Swamy. 1949. The Pasha, T. Zack & W. Hatley. 1986. Com nature and occurrence of septate fibres in puter-aided Wood Identification. Refer dicotyledons. Amer. 1. Bot. 36: 804 (ab ence Manual. North Carolina Agricultural stract). Research Service Bulletin 474. Sprague, T. 1906. Flora of Tropical Africa. Willis, J. C. 1973. A dictionary of the flower Vol. IV, Sect. 2, Hydrophyllaceae to. Pe ing plants. Revised by H. K. Airy Shaw. daliaceae. XCVI, Bignoniaceae: 512-538. 8th Ed. Cambridge Univ. Press. Steenis, C.G.G.J. van. 1977. Bignoniaceae. Wolkinger, F. 1970. Das Vorkommen leben In Flora Malesiana I, 8 (2): 114-186. der Holzfasem in Striiuchem und Bliumen. Sijthoff & Noordhoff, The Netherlands. Phyton (Austria) 14: 55-67. Stem, W. L. 1988. Index Xylariorum 3. In Zimmermann, M.H. 1983. Xylem structure stitutional wood collections of the world. and the ascent of sap. Springer Verlag, IAWA Bull. n.s. 9: 203-252. Berlin, Heidelberg, New York, Tokyo. APPENDIX The species examined are listed below. The country or geographical region of origin is that from which the specimen came, not necessarily its native habitat. If the exact source of the specimen is not known, but the native region is, this is in parentheses. -

Australian Marsupial Species Identification
G Model FSIGSS-793; No. of Pages 2 Forensic Science International: Genetics Supplement Series xxx (2011) xxx–xxx Contents lists available at ScienceDirect Forensic Science International: Genetics Supplement Series jo urnal homepage: www.elsevier.com/locate/FSIGSS Australian marsupial species identification a, b,e c,d d d Linzi Wilson-Wilde *, Janette Norman , James Robertson , Stephen Sarre , Arthur Georges a ANZPAA National Institute of Forensic Science, Victoria, Australia b Museum Victoria, Victoria, Australia c Australian Federal Police, Australian Capital Territory, Australia d University of Canberra, Australian Capital Territory, Australia e Melbourne University, Victoria, Australia A R T I C L E I N F O A B S T R A C T Article history: Wildlife crime, the illegal trade in animals and animal products, is a growing concern and valued at up to Received 10 October 2011 US$20 billion globally per year. Australia is often targeted for its unique fauna, proximity to South East Accepted 10 October 2011 Asia and porous borders. Marsupials of the order Diprotodontia (including koala, wombats, possums, gliders, kangaroos) are sometimes targeted for their skin, meat and for the pet trade. However, species Keywords: identification for forensic purposes must be underpinned by robust phylogenetic information. A Species identification Diprotodont phylogeny containing a large number of taxa generated from nuclear and mitochondrial Forensic data has not yet been constructed. Here the mitochondrial (COI and ND2) and nuclear markers (APOB, DNA IRBP and GAPD) are combined to create a more robust phylogeny to underpin a species identification COI Barcoding method for the marsupial order Diprotodontia. Mitochondrial markers were combined with nuclear Diprotodontia markers to amplify 27 genera of Diprotodontia. -

A Species-Level Phylogenetic Supertree of Marsupials
J. Zool., Lond. (2004) 264, 11–31 C 2004 The Zoological Society of London Printed in the United Kingdom DOI:10.1017/S0952836904005539 A species-level phylogenetic supertree of marsupials Marcel Cardillo1,2*, Olaf R. P. Bininda-Emonds3, Elizabeth Boakes1,2 and Andy Purvis1 1 Department of Biological Sciences, Imperial College London, Silwood Park, Ascot SL5 7PY, U.K. 2 Institute of Zoology, Zoological Society of London, Regent’s Park, London NW1 4RY, U.K. 3 Lehrstuhl fur¨ Tierzucht, Technical University of Munich, Alte Akademie 12, 85354 Freising-Weihenstephan, Germany (Accepted 26 January 2004) Abstract Comparative studies require information on phylogenetic relationships, but complete species-level phylogenetic trees of large clades are difficult to produce. One solution is to combine algorithmically many small trees into a single, larger supertree. Here we present a virtually complete, species-level phylogeny of the marsupials (Mammalia: Metatheria), built by combining 158 phylogenetic estimates published since 1980, using matrix representation with parsimony. The supertree is well resolved overall (73.7%), although resolution varies across the tree, indicating variation both in the amount of phylogenetic information available for different taxa, and the degree of conflict among phylogenetic estimates. In particular, the supertree shows poor resolution within the American marsupial taxa, reflecting a relative lack of systematic effort compared to the Australasian taxa. There are also important differences in supertrees based on source phylogenies published before 1995 and those published more recently. The supertree can be viewed as a meta-analysis of marsupial phylogenetic studies, and should be useful as a framework for phylogenetically explicit comparative studies of marsupial evolution and ecology. -

The Western Ringtail Possum (Pseudocheirus Occidentalis)
A major road and an artificial waterway are barriers to the rapidly declining western ringtail possum, Pseudocheirus occidentalis Kaori Yokochi BSc. (Hons.) This thesis is presented for the degree of Doctor of Philosophy of The University of Western Australia School of Animal Biology Faculty of Science October 2015 Abstract Roads are known to pose negative impacts on wildlife by causing direct mortality, habitat destruction and habitat fragmentation. Other kinds of artificial linear structures, such as railways, powerline corridors and artificial waterways, have the potential to cause similar negative impacts. However, their impacts have been rarely studied, especially on arboreal species even though these animals are thought to be highly vulnerable to the effects of habitat fragmentation due to their fidelity to canopies. In this thesis, I studied the effects of a major road and an artificial waterway on movements and genetics of an endangered arboreal species, the western ringtail possum (Pseudocheirus occidentalis). Despite their endangered status and recent dramatic decline, not a lot is known about this species mainly because of the difficulties in capturing them. Using a specially designed dart gun, I captured and radio tracked possums over three consecutive years to study their movement and survival along Caves Road and an artificial waterway near Busselton, Western Australia. I studied the home ranges, dispersal pattern, genetic diversity and survival, and performed population viability analyses on a population with one of the highest known densities of P. occidentalis. I also carried out simulations to investigate the consequences of removing the main causes of mortality in radio collared adults, fox predation and road mortality, in order to identify effective management options. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant.