Thesis Proposal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study of Fragments of Mural Paintings from the Roman Province Of
Study of fragments of mural paintings from the Roman province of Germania Superior Thesis submitted in partial fulfilment of the requirements of the degree Doctor rer. nat. of the Faculty of Environment and Natural Resources, Albert-Ludwigs-Universität Freiburg im Breisgau, Germany by Rafaela Debastiani Freiburg im Breisgau, Germany 2016 Name of Dean: Prof. Dr. Tim Freytag Name of Supervisor: Prof. Dr. Michael Fiederle Name of 2nd Reviewer: PD Dr. Andreas Danilewsky Date of thesis' defense: 03.02.2017 “The mind is not a vessel to be filled, but a fire to be kindled” Plutarch Contents Nomenclature ........................................................................................................................ 1 Acknowledgment ................................................................................................................... 3 Abstract ................................................................................................................................. 5 Zusammenfassung ................................................................................................................ 7 1. Introduction .................................................................................................................... 9 2. Analytical techniques in the non-destructive analyses of fragments of mural paintings ..13 2.1 X-ray Fluorescence Spectroscopy ..........................................................................13 2.1.1 Synchrotron-based scanning macro X-ray fluorescence (MA-XRF) .................17 -

Gamblin Provides Is the Desire to Help Painters Choose the Materials That Best Support Their Own Artistic Intentions
AUGUST 2008 Mineral and Modern Pigments: Painters' Access to Color At the heart of all of the technical information that Gamblin provides is the desire to help painters choose the materials that best support their own artistic intentions. After all, when a painting is complete, all of the intention, thought, and feeling that went into creating the work exist solely in the materials. This issue of Studio Notes looks at Gamblin's organization of their color palette and the division of mineral and modern colors. This visual division of mineral and modern colors is unique in the art material industry, and it gives painters an insight into the makeup of pigments from which these colors are derived, as well as some practical information to help painters create their own personal color palettes. So, without further ado, let's take a look at the Gamblin Artists Grade Color Chart: The Mineral side of the color chart includes those colors made from inorganic pigments from earth and metals. These include earth colors such as Burnt Sienna and Yellow Ochre, as well as those metal-based colors such as Cadmium Yellows and Reds and Cobalt Blue, Green, and Violet. The Modern side of the color chart is comprised of colors made from modern "organic" pigments, which have a molecular structure based on carbon. These include the "tongue- twisting" color names like Quinacridone, Phthalocyanine, and Dioxazine. These two groups of colors have unique mixing characteristics, so this organization helps painters choose an appropriate palette for their artistic intentions. Eras of Pigment History This organization of the Gamblin chart can be broken down a bit further by giving it some historical perspective based on the three main eras of pigment history – Classical, Impressionist, and Modern. -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Textures of Williamsburg Handmade Oil Colors
Textures of Williamsburg Handmade Oil Colors VERY FINE FINE MEDIUM COARSE Alizarin Orange Permanent Red-Orange Brown Umber Alizarin Crimson Brown Pink Bismuth Vanadate Yellow Permanent Yellow Deep Burnt Sienna Alizarin Yellow Dutch Brown (Transparent) Brilliant Yellow Extra Pale Permanent Yellow Light Burnt Umber Bohemian Green Earth Italian Pink Brilliant Yellow Pale Permanent Yellow Medium Cadmium Green Brown Ochre Olive Green Carl’s Crimson Persian Rose Cadmium Green Light Cyprus Orange Stil De Grain Cerulean Blue French Phthalo Blue Cadmium Lemon Earth Green Chromium Oxide Green Phthalo Green Cadmium Orange French Ardoise Grey Cinnabar Green Light Phthalo Green-Yellowish Cadmium Purple French Brown Ochre Cobalt Blue Phthalo Turquoise Cadmium Red Deep French Burnt Ochre Cobalt Blue Deep Provence Violet Bluish Cadmium Red Light French Burnt Umber Cobalt Green Provence Violet Reddish Cadmium Red Medium French Light Sienna Cobalt Teal Bluish Prussian Blue Cadmium Red Purple French Ochre Havane Cobalt Teal Greenish Pyrrole Orange Cadmium Red Vermilion French Raw Sienna Cobalt Turquoise Bluish Pyrrole Red Cadmium Yellow Deep French Raw Umber Cobalt Turquoise Greenish Quinacridone Magenta Cadmium Yellow Extra Deep French Rouge Indien Cobalt Violet Deep Quinacridone Red Cadmium Yellow Light French Terre Verte Cobalt Violet Light Quinacridone Violet Cadmium Yellow Medium French Yellow Ochre Deep Courbet Green Sevres Blue Canton Rose German Earth Dianthus Pink SF Cerulean Blue French Cerulean Blue (Genuine) Graphite Grey Egyptian Violet SF -

Paint Pigments— Yellow
» TECHNICAL INFORMATION ON BUILDING MATERIALS TIBM - 32 FOR UfSE IN THE DESIGN OF LOW-COST HOUSING ***** THE NATIONAL BUREAU OF STANDARDS UNITED STATES DEPARTMENT OF COMMERCE WASHINGTON, D. C. August 29, 1936 PAINT PIGMENTS— YELLOW, . BROWN, BLUE, GREEN, AND BRONZE This is urimarily^a digest of the sections of Bureau of Standards Circular No, o9> "Paint and Varnish", (November 17, 1917),'*' and Tech- nologic Paper No. 274, "Use of United States Government Suecif ication Paints and Faint Materials", (December 15, 1924), ^ Ly p, H. Walker and E. F. Hickson, dealing with general composition , characteristics, and uses of yellow, brown, blue, green, and bronze pigments. The following papers contain additional information relative to paint pigments, oil paints, and water paints: TIBM - 30 "Paint Pigments—White" TIBM - 31 "Paint Pigments—Black, Red, and Lakes" TIBM - 33 "Federal Specification . Paint Pigments and Mixing Formulas" TIM - 3U "Federal Specification Ready-Mixed Paints, Semi- paste Paints and Mixing Formulas’"' TIBM - 35 "Preparation of Paints from Paste and Dry Pigments" TIBM - 36 "Preparation of Paints from Semipaste Paints, Thinning Ready-Mixed Paints, and Preparation of Water Paints" TIBM - 43 "Aluminum Paints" Pigments are "the fine solid warticles used in the preparation of paint, and substantially insoluble in the vehicle, "3 In general, it may be ^Out of print. May be consulted in Government depositor}*- libraries. p Available from Superintendent of Documents, Government Printing Office, Washington, D. C. .(Price 10 cents). ^Qpioted from "Standard Definitions of Terms Relating to Paint 'Specifications", American Society for Testing Materials ( 1 93 3 ) ’ • • -• •• PP. 735-73 9 . 031736-C - 1 - assumed that pigments composed of very fine particles, having high re- fractive indices, provide the greatest covering power and opacity. -

Stereum Species (Stereaceae) of South Africa
)0 s. Mr. 1. Bot. 1998, 64( 1130- 37 Stereum species (Stereaceae) of South Africa Albert Eicker* and Sandra Louw Department of Botany, University of Pretoria, Pretoria 0002 Republic of South Africa Received 3 June 1997; revised 20 August J 997 Seven species of Stereum HiIl:Pers. are recognised for South Africa. Three species, S. hirsutum, (Willd,:Fr.) Gray, S. rimosum Berk. and S. ochraceo-flavum (Schwein.) Ellis, have only ordinary hyphidia and belong to the subgenus Stereum. S. rimosum var africanum P.H.B. Talbot is reduced to synonymy with S. rimosum. S. vel/ereum Berkeley is reduced to synonymy with S. ochraceo-flavum. S. lobatum (Kunze:Fr.) Fr., S. australe Lloyd and S. sanguino/entum (Alb. & Schwein .:Fr.) Fr. , possess pseudoacanthohyphidia and therefore belong to the subgenus Aculeatostereum. Representatives of the S. ostrea complex in South Africa are S. lobatum and S. austra/e, both considered well ~ defined species. S. durbanense van der Sijl is considered a synonym of S. australe. S. illudens Berk. is the only species recognised in th is study as possessing acanthohyphidia and therefore belongs to the subgenus Acanthastereum. A dichotomous key based on micro- and macromorphological characters is given for the seven South African species. Keywords: Stereum, S. austra/e, S. hirsutum, S. illudens, S. fobatum, S. ochraceo-flavum. S. rimosum, S. sanguinolentum, South African fungi. *To whom correspondence shou ld be add ressed. Introduction be of great importance in the taxonomy of this genus. had either Talbot's (1954) concept of the genus Siereum in South Africa not been described or their importance had not been appreciated was analogous with that of Burt (1920) and included 'stereoid' when Talbot (1954) wrote his paper on South African stereums. -
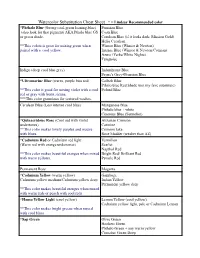
Watercolor Substitution Cheat Sheet * = Lindsay Recommended Color
Watercolor Substitution Cheat Sheet * = Lindsay Recommended color *Phthalo Blue (Strong cool-green leaning-blue) Prussian Blue (also look for that pigment) AKA Pthalo blue GS Cyan Blue or green shade. Cerulean Blue (if it looks dark: Mission Gold) Helio Cerulean **This colors is great for mixing green when Winsor Blue (Winsor & Newton) paired with a cool yellow. Intense Blue (Winsor & Newton/Cotman) Azure (Yarka/White Nights) Turquoise Indigo (deep cool blue grey) Indanthrone Blue Payne's Grey+Prussian Blue *Ultramarine Blue (warm, purple bias red) Colbalt Blue Pthalo blue Red Shade (not my fave substitute) **This color is good for mixing violet with a cool Poland Blue red or gray with burnt sienna. ***This color granulates for textured washes. Cerulean Blue (Less intense cool blue) Manganese Blue Phthalo blue + white Cinerous Blue (Sennelier) *Quinacridone Rose (Cool red with violet Alizarian Crimson undertones) Carmine **This color makes lovely purples and mauve Crimson lake with blues. Rose Madder (weaker than AZ) *Cadmium Red or Cadmium red light Vermilion (Warm red with orange undertones) Scarlet Napthol Red **This color makes beautiful oranges when mixed Bright Red/ Brilliant Red with warm yellows. Pyrrole Red Permanent Rose Magenta *Cadmium Yellow (warm yellow) Gamboge Cadmium yellow medium/Cadmium yellow deep Indian Yellow Permanent yellow deep **This color makes beautiful oranges when mixed with warm reds or peach with cool reds *Hansa Yellow Light (cool yellow) Lemon Yellow (cool yellow) Cadmium yellow light, pale or Cadmium -

EF MAXOPAKE SIENNA BROWN Product Overview Product Code PADE7031 Instructions Industry Inks Dark Garments
Recommendations EF MAXOPAKE SIENNA BROWN Product Overview Product Code PADE7031 Instructions Industry Inks Dark garments. Direct printing. 100% cotton or cotton/polyester.* Stencils: Use any direct emulsion or Application Screen Printing capillary film compatible with plastisol inks. Additives: Maxopake inks are supplied ready to print. If necessary to reduce viscosity, use up to 15% by weight, of Reducer/Detackifi er (PLRE-9000). For printing Category Stock Colors transfers, mix Maxopake with with 5-10% Hot Split Additive (PLUE-9040). Printing Instructions: Multiple Chemistry Plastisol strokes may be required when printing by hand. When printing with automatic presses use a slightly rounded squeegee to print a thicker ink layer. A soft pad on the printing pallet will minimize penetration Substrate(s) Cotton into the garment and improve opacity. Curing Instructions: These inks will fully cure when the entire Best Used By 12 months thickness of the ink deposit reaches 300°•F (149°•C). Using Low-Bleed Inks: The Maxopake series includes two low-bleed colors: Low-Bleed Medium Yellow (PADE-2060), and Low-Bleed Golden Yellow Certification(s) ISO9001 (PADE-2048). The lowbleed inks are recommended for printing on cotton/polyester garments to control Performance: the problem of dyes in the polyester fi bers migrating or _ã–bleeding_ã• into the plastisol ink. Low-bleed inks are not recommended for printing on light-colored 100% cotton fabrics. On rare occasions ghost After Flash Tack Decreases with increased images can appear on contacting surfaces of ink to garment. The use of low-bleed plastisols on these mesh fabrics is not recommended. If low-bleed colors are used, be sure to fully cure, to minimize the possibility Squeegee: of ghost images. -

Ascochyta Manawaorae Persoonial Reflections 129
128 Persoonia – Volume 24, 2010 Ascochyta manawaorae Persoonial Reflections 129 Fungal Planet 45 – 18 June 2010 Ascochyta manawaorae Verkley, Woudenberg & De Gruyter, sp. nov. Teleomorph. Unknown (anamorphic Phaeosphaeriaceae, based on Typus. NEW ZEALAND, North Island, Northland, Bay of Islands area, Mana- molecular analysis). waora near Russell, on dead leaves and stems of Salicornia australis, on the border of a mangrove vegetation, 30 Jan. 2003, G. Verkley 2022b; PDD Conidiomata pycnidialia, superficialia vel epidermide erumpentia, globosa, 98412 holotype, culture ex-type CBS 117477 = ICMP 18292, ITS sequence fusca vel atra, 100–200(–250) µm diam; ostiolum centrale, circulare, 10–15 GenBank GU230751, MycoBank MB497140. µm diam. Cellulae conidiogenae discretae, determinatae, holoblasticae, inter- dum percurrentes et obscure annulatae, doliiformae vel breve ampulliformae, Notes — No teleomorph was observed that could be as- 3–5 × 4–7(–9) µm; conidia cylindrica, in medio septata, rare 2–3-septata, sociated with A. manawaorae. Based on ITS rDNA analysis, pallide lutescens vel olivacescens, 12–19 × 2–3 µm. the genetically closest teleomorphs are members of the genus Etymology. Named after the village of Manawaora near the type locality, Phaeosphaeria (Phaeosphaeriaceae, type species P. oryzae). Bay of Islands, New Zealand. Our fungus is morphologically close to species that have been described in the coelomycete genus Ascochyta. Although it is Conidiomata (in vivo) pycnidial, superficial or erumpent from the not closely related to A. pisi, the type species of the genus Asco epidermis, globose, dark brown to black, 100–200(–250) µm chyta, it is described here in this genus pending further work diam; ostiole central, circular, 10–15 µm diam, surrounded by to resolve the various lineages of Ascochyta-like anamorphs, dark brown, thick-walled cells; pycnidial wall composed of three for which new generic names need to ultimately be proposed. -

MSDS for #02807 - PIGMENT SET Page 1 of 5
MSDS for #02807 - PIGMENT SET Page 1 of 5 02807-1159 SAFETY DATA SHEET Section 1: Product and Company Indentification Sinopia INC PO BOX 4884354 San Francisco, CA 94188 Tel. (415) 824-3180 Fax (415) 800-6987 Chemical Emergency Hotline 1 800 424 9300 Product Name: Sinopia Complete Pigments Set Revision Date: 04/18 Section 2: Hazards Identification Section 3: Composition/Information on Ingredients Ingredients CAS# Hansa Yellow 6448-95-9 Yellow Ochre, golden 1343-81-3 Yellow Ochre, MEXICO 1343-81-3 Raw Sienna, deep orange 1317-63-1 Napthol Red 6448-95-9 English Red 1309-37-1 Burnt Sienna 1317-63-1 Turkish Red 1309-37-1 Roman Brown 1309-37-1 Burnt Umber, 12713-03-0 Raw Umber 12713-03-0 Chrome Oxide Green 1308-38-9 PB15, Phtalo Blue 147-14-8 Titanium White, Rutile 13463-67-7 Item Numbers: 02807-1159 Page 1 of 5 MSDS for #02807 - PIGMENT SET Page 2 of 5 Section 4: First Aid Measures Acute Toxicity LD50/oral /rat: >5000 mg/kg body weightLD50/DERMAL IRRITATION-RABBIT: Non-irritating EYE IRRITATION-RABBIT: Non-irritating EXEPCTED ROUTE OF ENTRY: Skin contact – Skin absorption – Eye contact -Inhalation - Ingestion EFFECTS OF EXPOSURE: No toxicological test data is available and no information was found in the available literature for any effects of exposure: No health evaluation is thus possible, handle with care and avoid unnecessary exposures. Section 5: Fire-Fighting Measures Suitable Extinguishing Media: This product will not burn. Use suitable extinguishing media for fighting surrounding fire. Unsuitable Extinguishing Media: None Applicable Emitted when burned: None Applicable Special protective equipment: This product will not burn. -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin