Recovery of Functional Connectivity of the Sensorimotor Network After Surgery for Diffuse Low-Grade Gliomas Involving the Supplementary Motor Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring the Sensorimotor Network Using Functional Connectivity and Graph Theory
EXPLORING THE SENSORIMOTOR NETWORK USING FUNCTIONAL CONNECTIVITY AND GRAPH THEORY by Ronald Bishop Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia August 2014 © Copyright by Ronald Bishop, 2014 TABLE OF CONTENTS LIST OF TABLES ......................................................................................................................................... v LIST OF FIGURES ...................................................................................................................................... vi ABSTRACT ................................................................................................................................................. vii LIST OF ABBREVIATIONS USED ......................................................................................................... viii ACKNOWLEDGEMENTS ......................................................................................................................... xi CHAPTER 1: INTRODUCTION ................................................................................................................. 1 CHAPTER 2: LITERATURE REVIEW ..................................................................................................... 4 2.1 AN OVERVIEW OF NEURONAL COMMUNICATION ..................................................................................... 4 2.2 SYNCHRONOUS NEURAL FIRING .............................................................................................................. -
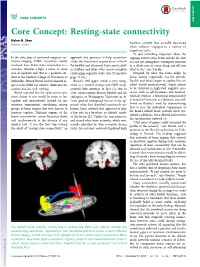
Core Concept: Resting-State Connectivity Helen H
CORE CONCEPTS CORE CONCEPTS Core Concept: Resting-state connectivity Helen H. Shen baseline activity that actually decreased Science Writer when subjects engaged in a variety of cognitive tasks. “It said something important about the In the early days of functional magnetic res- approach that promises to help researchers ongoing activity of the brain, and the fact that onance imaging (fMRI), researchers mostly study the functional organization of both it is not just sitting there waiting for someone analyzed how brain areas responded to a the healthy and abnormal brain, particularly in a white coat to come along and tell you stimulus, whether a light, a noise, or some in children and others who cannot complete what to do,” says Raichle. sort of cognitive task. But as a graduate stu- challenging cognitive tasks. (See Perspective Intrigued by what the brain might be dent at the Medical College of Wisconsin in page 14105.) doing during supposedly inactive periods, Milwaukee, Bharat Biswal had an unusual re- Biswal’s 1995 paper, which is now recog- Raichle and others began to explore this so- quest of his fMRI test subjects: climb into the nized as a seminal resting-state fMRI study, called “default mode network,” which seemed scanner and do, well, nothing. received little attention at first (1). But in to be involved in high-level cognitive pro- Biswal expected that the spontaneous neu- 2001, neuroscientist Marcus Raichle and his cesses, such as self-awareness and memory. ronal chatter at rest would be more or less colleagues at Washington University in St. Michael Greicius, a behavioral neuroscientist random and unstructured. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Functional Role of the Supplementary and Pre-Supplementary Motor Areas
REVIEWS Functional role of the supplementary and pre-supplementary motor areas Parashkev Nachev*‡, Christopher Kennard‡ and Masud Husain* Abstract | The supplementary motor complex consists of the supplementary motor area, the supplementary eye field and the pre-supplementary motor area. In recent years, these areas have come under increasing scrutiny from cognitive neuroscientists, motor physiologists and clinicians because they seem to be crucial for linking cognition to action. However, theories regarding their function vary widely. This Review brings together the data regarding the supplementary motor regions, highlighting outstanding issues and providing new perspectives for understanding their functions. Ever since they were first identified, the regions that which we hope will stimulate the development of comprise the supplementary motor complex (SMC) have conceptual frameworks for a better understanding remained, in large part, a mystery. Most investigators now of this region. appreciate that these brain regions are far from ‘supple- mentary’ to requirements1–7. Without them, there are Anatomy and connections profound alterations in behaviour. For example, lesions to The supplementary motor area (SMA) and pre- these areas in humans can lead to alien-limb syndrome, supplementary motor area (pre-SMA) are, in humans, with patients demonstrating involuntary actions such as located on the medial aspect of the brain: in the dorso- grasping nearby objects — even other people — without medial frontal cortex3,14, anterior to the leg representa- ever intending to do so8,9. Some individuals demonstrate tion of the primary motor cortex (FIG. 1). Both areas lie utilization behaviour: unable to resist the impulse to use in the superior frontal gyrus and constitute the medial an object that has been placed within their reach, even part of Brodmann’s area 6c (later divided into two areas: when the object is not needed10. -

Stimulus Arousal Drives Amygdalar Responses to Emotional
www.nature.com/scientificreports OPEN Stimulus arousal drives amygdalar responses to emotional expressions across sensory modalities Huiyan Lin1,2,4*, Miriam Müller-Bardorf2,4, Bettina Gathmann2,4, Jaqueline Brieke2, Martin Mothes-Lasch2, Maximilian Bruchmann2, Wolfgang H. R. Miltner3 & Thomas Straube2 The factors that drive amygdalar responses to emotionally signifcant stimuli are still a matter of debate – particularly the proneness of the amygdala to respond to negatively-valenced stimuli has been discussed controversially. Furthermore, it is uncertain whether the amygdala responds in a modality-general fashion or whether modality-specifc idiosyncrasies exist. Therefore, the present functional magnetic resonance imaging (fMRI) study systematically investigated amygdalar responding to stimulus valence and arousal of emotional expressions across visual and auditory modalities. During scanning, participants performed a gender judgment task while prosodic and facial emotional expressions were presented. The stimuli varied in stimulus valence and arousal by including neutral, happy and angry expressions of high and low emotional intensity. Results demonstrate amygdalar activation as a function of stimulus arousal and accordingly associated emotional intensity regardless of stimulus valence. Furthermore, arousal-driven amygdalar responding did not depend on the visual and auditory modalities of emotional expressions. Thus, the current results are consistent with the notion that the amygdala codes general stimulus relevance across visual -

Translingual Neural Stimulation with the Portable Neuromodulation
Translingual Neural Stimulation With the Portable Neuromodulation Stimulator (PoNS®) Induces Structural Changes Leading to Functional Recovery In Patients With Mild-To-Moderate Traumatic Brain Injury Authors: Jiancheng Hou,1 Arman Kulkarni,2 Neelima Tellapragada,1 Veena Nair,1 Yuri Danilov,3 Kurt Kaczmarek,3 Beth Meyerand,2 Mitchell Tyler,2,3 *Vivek Prabhakaran1 1. Department of Radiology, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin, USA 2. Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA 3. Department of Kinesiology, University of Wisconsin-Madison, Madison, Wisconsin, USA *Correspondence to [email protected] Disclosure: Dr Tyler, Dr Danilov, and Dr Kaczmarek are co-founders of Advanced Neurorehabilitation, LLC, which holds the intellectual property rights to the PoNS® technology. Dr Tyler is a board member of NeuroHabilitation Corporation, a wholly- owned subsidiary of Helius Medical Technologies, and owns stock in the corporation. The other authors have declared no conflicts of interest. Acknowledgements: Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, Ashfield Healthcare Communications, part of UDG Healthcare plc, funded by Helius Medical Technologies. Dr Tyler, Dr Kaczmarek, Dr Danilov, Dr Hou, and Dr Prabhakaran were being supported by NHC-TBI-PoNS-RT001. Dr Hou, Dr Kulkarni, Dr Nair, Dr Tellapragada, and Dr Prabhakaran were being supported by R01AI138647. Dr Hou and Dr Prabhakaran were being supported by P01AI132132, R01NS105646. Dr Kulkarni was being supported by the Clinical & Translational Science Award programme of the National Center for Research Resources, NCATS grant 1UL1RR025011. Dr Meyerand, Dr Prabhakaran, Dr Nair was being supported by U01NS093650. -

Short- and Long-Term Deficits After Unilateral Ablation Andthe Effects of Subsequent Callosal Section’
0270.6474/84/0404-0918$02.00/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 4, pp. 918-929 Printed in U.S.A. April 1984 SUPPLEMENTARY MOTOR AREA OF THE MONKEY’S CEREBRAL CORTEX: SHORT- AND LONG-TERM DEFICITS AFTER UNILATERAL ABLATION ANDTHE EFFECTS OF SUBSEQUENT CALLOSAL SECTION’ COBIE BRINKMAN Experimental Neurology Unit, The John Curtin School of Medical Research, Australian National University, Canberra, Australia 2601 Received March 7, 1983; Revised November 11, 1983; Accepted November 15, 1983 Abstract The short-term and long-term behavioral effects of unilateral lesions of the supplementary motor area (SMA) were studied in five monkeys (Mczcaca fascicularis ssp). A monkey with a unilateral lesion of the premotor area (PM) served as a control. In all animals, general behavior was unaffected by the lesions. For a few weeks postoperatively, all monkeys showed a clumsiness of forelimb movements, bilaterally, which involved both the distal and proximal muscles. Two SMA-lesioned monkeys (but not the PM-lesioned one), studied for up to 1 year postoperatively, showed a characteristic deficit of bimanual coordination where the two hands tended to behave in a similar manner instead of sharing the task between them. This deficit was more pronounced after a lesion contralateral to the nonpreferred hand. The deficit was interpreted as indicating that the intact SMA now influenced the motor outflow of both the ipsilateral hemisphere and the contralateral one through the corpus callosum. Callosal section immediately abolished the bimanual deficit, although the clumsiness returned transiently. The results imply that SMA may give rise normally to discharges informing the contralateral hemisphere of intended and/or ongoing movements via the corpus callosum. -

Down but Not out in Posterior Cingulate Cortex: Deactivation Yet Functional Coupling with Prefrontal Cortex During Demanding Semantic Cognition
NeuroImage 141 (2016) 366–377 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition Katya Krieger-Redwood ⁎, Elizabeth Jefferies, Theodoros Karapanagiotidis, Robert Seymour, Adonany Nunes, Jit Wei Aaron Ang, Vierra Majernikova, Giovanna Mollo, Jonathan Smallwood Department of Psychology/York Neuroimaging Centre, University of York, Heslington, York, United Kingdom article info abstract Article history: The posterior cingulate cortex (pCC) often deactivates during complex tasks, and at rest is often only weakly cor- Received 30 March 2016 related with regions that play a general role in the control of cognition. These observations led to the hypothesis Accepted 29 July 2016 that pCC contributes to automatic aspects of memory retrieval and cognition. Recent work, however, has sug- Available online 30 July 2016 gested that the pCC may support both automatic and controlled forms of memory processing and may do so by changing its communication with regions that are important in the control of cognition across multiple do- Keywords: mains. The current study examined these alternative views by characterising the functional coupling of the Posterior cingulate cortex Dorsolateral prefrontal cortex pCC in easy semantic decisions (based on strong global associations) and in harder semantic tasks (matching Semantic control words on the basis of specific non-dominant features). Increasingly difficult semantic decisions led to the expect- Executive ed pattern of deactivation in the pCC; however, psychophysiological interaction analysis revealed that, under Rest these conditions, the pCC exhibited greater connectivity with dorsolateral prefrontal cortex (PFC), relative to Connectivity both easier semantic decisions and to a period of rest. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Neuroimage: Clinical 15 (2017) 513–524
NeuroImage: Clinical 15 (2017) 513–524 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Disruption to control network function correlates with altered dynamic MARK connectivity in the wider autism spectrum ⁎ N. de Lacya,b, D. Dohertyb,c, B.H. Kingd, S. Rachakondae, V.D. Calhoune,f, a Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195, USA b Seattle Children's Research Institute, Center for Integrative Brain Research, Seattle, WA 98105, USA c Department of Pediatrics, Divisions of Developmental and Genetic Medicine, University of Washington, Seattle, WA 98195, USA d Department of Psychiatry, University of California San Francisco, San Francisco, CA 94143, USA e The Mind Research Network & LBERI, Albuquerque, NM 87106, USA f Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM 87131, USA ARTICLE INFO ABSTRACT Keywords: Autism is a common developmental condition with a wide, variable range of co-occurring neuropsychiatric Autism symptoms. Contrasting with most extant studies, we explored whole-brain functional organization at multiple Functional MRI levels simultaneously in a large subject group reflecting autism's clinical diversity, and present the first network- Dynamic connectivity based analysis of transient brain states, or dynamic connectivity, in autism. Disruption to inter-network and inter- Control networks system connectivity, rather than within individual networks, predominated. We identified coupling disruption in Task-positive the anterior-posterior default mode axis, and among specific control networks specialized for task start cues and Default-mode the maintenance of domain-independent task positive status, specifically between the right fronto-parietal and cingulo-opercular networks and default mode network subsystems. -

Development of Thalamocortical Connectivity During Infancy and Its Cognitive Correlations
The Journal of Neuroscience, July 2, 2014 • 34(27):9067–9075 • 9067 Development/Plasticity/Repair Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations Sarael Alcauter,1 Weili Lin,1 X J. Keith Smith,2 Sarah J. Short,3 Barbara D. Goldman,4 J. Steven Reznick,4 John H. Gilmore,3 and Wei Gao1 1Department of Radiology and Biomedical Research Imaging Center, 2Department of Radiology, 3Department of Psychiatry, and 4Frank Porter Graham Child Development Institute and Department of Psychology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599 Although commonly viewed as a sensory information relay center, the thalamus has been increasingly recognized as an essential node in various higher-order cognitive circuits, and the underlying thalamocortical interaction mechanism has attracted increasing scientific interest. However, the development of thalamocortical connections and how such development relates to cognitive processes during the earliest stages of life remain largely unknown. Leveraging a large human pediatric sample (N ϭ 143) with longitudinal resting-state fMRI scans and cognitive data collected during the first 2 years of life, we aimed to characterize the age-dependent development of thalamo- cortical connectivity patterns by examining the functional relationship between the thalamus and nine cortical functional networks and determine the correlation between thalamocortical connectivity and cognitive performance at ages 1 and 2 years. Our results revealed that the thalamus–sensorimotor -

Opposite Effects of Dopamine and Serotonin on Resting-State Networks: Review and Implications for Psychiatric Disorders
Molecular Psychiatry https://doi.org/10.1038/s41380-019-0406-4 REVIEW ARTICLE Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders 1,2 3 1,2,4,5 1,2 2,6,7 Benedetta Conio ● Matteo Martino ● Paola Magioncalda ● Andrea Escelsior ● Matilde Inglese ● 1,2 8,9,10 Mario Amore ● Georg Northoff Received: 15 September 2018 / Revised: 18 January 2019 / Accepted: 5 March 2019 © Springer Nature Limited 2019 Abstract Alterations in brain intrinsic activity—as organized in resting-state networks (RSNs) such as sensorimotor network (SMN), salience network (SN), and default-mode network (DMN)—and in neurotransmitters signaling—such as dopamine (DA) and serotonin (5-HT)—have been independently detected in psychiatric disorders like bipolar disorder and schizophrenia. Thus, the aim of this work was to investigate the relationship between such neurotransmitters and RSNs in healthy, by reviewing the relevant work on this topic and performing complementary analyses, in order to better understand their physiological link, as well as their alterations in psychiatric disorders. According to the reviewed data, neurotransmitters nuclei diffusively project to 1234567890();,: 1234567890();,: subcortical and cortical regions of RSNs. In particular, the dopaminergic substantia nigra (SNc)-related nigrostriatal pathway is structurally and functionally connected with core regions of the SMN, whereas the ventral tegmental area (VTA)-related mesocorticolimbic pathway with core regions of the SN. The serotonergic raphe nuclei (RNi) connections involve regions of the SMN and DMN. Coherently, changes in neurotransmitters activity impact the functional configuration and level of activity of RSNs, as measured by functional connectivity (FC) and amplitude of low-frequency fluctuations/temporal variability of BOLD signal.