Impaired Effective Functional Connectivity of the Sensorimotor Network in Interictal Episodic Migraineurs Without Aura
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accelerated Aging of Functional Brain Networks Supporting Cognitive Function in Psychotic Disorders
Biological Psychiatry: Celebrating 50 Years Archival Report Accelerated Aging of Functional Brain Networks Supporting Cognitive Function in Psychotic Disorders Julia M. Sheffield, Baxter P. Rogers, Jennifer U. Blackford, Stephan Heckers, and Neil D. Woodward ABSTRACT BACKGROUND: Across networks, connectivity within the frontoparietal network (FPN) and cingulo-opercular network (CON) exhibits reductions earliest during healthy aging, contributing to cognitive impairment. Individuals with psychotic disorders demonstrate evidence of accelerated aging across multiple biological systems. By leveraging a large sample of patients with psychosis from early to chronic illness stages, this study sought to determine whether the CON and FPN exhibit evidence of accelerated aging in psychotic disorders, confirm associations between network efficiency and cognition, and determine whether reduced network efficiency is observed in early-stage psychosis. METHODS: Resting-state functional magnetic resonance imaging and cognitive data were obtained on 240 patients with psychotic disorder and 178 healthy control participants (HCs). Global efficiency, a measure of functional inte- gration, was calculated for the CON, FPN, subcortical network, and visual network. Associations with age and cognition were assessed and compared between groups. RESULTS: Consistent with accelerated aging, significant group by age interactions reflected significantly stronger relationships between efficiency and age in patients with psychosis than in HCs for both the CON (psychosis: r = 2.37; HC: r = 2.16) and FPN (psychosis: r = 2.31; HC: r = 2.05). Accelerated aging was not observed in either the subcortical or visual network, suggesting specificity for cognitive networks that decline earliest in healthy aging. Replicating prior findings, efficiency of both the CON and FPN correlated with cognitive function across all partici- pants (rs . -

Exploring the Sensorimotor Network Using Functional Connectivity and Graph Theory
EXPLORING THE SENSORIMOTOR NETWORK USING FUNCTIONAL CONNECTIVITY AND GRAPH THEORY by Ronald Bishop Submitted in partial fulfillment of the requirements for the degree of Master of Science at Dalhousie University Halifax, Nova Scotia August 2014 © Copyright by Ronald Bishop, 2014 TABLE OF CONTENTS LIST OF TABLES ......................................................................................................................................... v LIST OF FIGURES ...................................................................................................................................... vi ABSTRACT ................................................................................................................................................. vii LIST OF ABBREVIATIONS USED ......................................................................................................... viii ACKNOWLEDGEMENTS ......................................................................................................................... xi CHAPTER 1: INTRODUCTION ................................................................................................................. 1 CHAPTER 2: LITERATURE REVIEW ..................................................................................................... 4 2.1 AN OVERVIEW OF NEURONAL COMMUNICATION ..................................................................................... 4 2.2 SYNCHRONOUS NEURAL FIRING .............................................................................................................. -
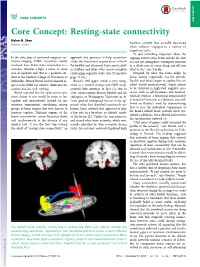
Core Concept: Resting-State Connectivity Helen H
CORE CONCEPTS CORE CONCEPTS Core Concept: Resting-state connectivity Helen H. Shen baseline activity that actually decreased Science Writer when subjects engaged in a variety of cognitive tasks. “It said something important about the In the early days of functional magnetic res- approach that promises to help researchers ongoing activity of the brain, and the fact that onance imaging (fMRI), researchers mostly study the functional organization of both it is not just sitting there waiting for someone analyzed how brain areas responded to a the healthy and abnormal brain, particularly in a white coat to come along and tell you stimulus, whether a light, a noise, or some in children and others who cannot complete what to do,” says Raichle. sort of cognitive task. But as a graduate stu- challenging cognitive tasks. (See Perspective Intrigued by what the brain might be dent at the Medical College of Wisconsin in page 14105.) doing during supposedly inactive periods, Milwaukee, Bharat Biswal had an unusual re- Biswal’s 1995 paper, which is now recog- Raichle and others began to explore this so- quest of his fMRI test subjects: climb into the nized as a seminal resting-state fMRI study, called “default mode network,” which seemed scanner and do, well, nothing. received little attention at first (1). But in to be involved in high-level cognitive pro- Biswal expected that the spontaneous neu- 2001, neuroscientist Marcus Raichle and his cesses, such as self-awareness and memory. ronal chatter at rest would be more or less colleagues at Washington University in St. Michael Greicius, a behavioral neuroscientist random and unstructured. -

514414V1.Full.Pdf
bioRxiv preprint doi: https://doi.org/10.1101/514414; this version posted January 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running title: Primary somatosensory cortex connections. Title: Afferent connections of the primary somatosensory cortex of the mouse for contextual and multisensory processing. Author: Ian Omer Massé PhD1, Sohen Blanchet-Godbout2, Gilles Bronchti PhD2, Denis Boire PhD2 Affiliations: details 1Research Center, Hôpital du Sacré-Cœur de Montréal,for 5400 Gouin Ouest Blvd, Montreal, Quebec, Canada, H4J 1C5 DOI 2Département d’Anatomie, Université du Québec à Trois-Rivières, 3351, des Forges Blvd, C.P. 500, Trois-Rivières, Quebec, Canada, G9A 2W7 manuscript WITHDRAWNsee Number of pages: Number of figures: Number of tables: Number of equations: Total number of words: Number of words in abstract: Keywords: Cross-modal, corticocortical connections, subcortical connections, feedforward, feedback, top-down, bottom-up Corresponding author: Ian Omer Massé PhD Centre de recherche Hôpital du Sacré-Cœur de Montréal 5400, boulevard Gouin Ouest Montréal, Québec H4J 1C5 Phone: 514-338-2222 ext 7711 FAX: 514 338-2694 E-mail address: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/514414; this version posted January 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

Childhood Trauma History Is Linked to Abnormal Brain Connectivity in Major Depression
Childhood trauma history is linked to abnormal brain connectivity in major depression Meichen Yua,b, Kristin A. Linna,c, Russell T. Shinoharaa,c, Desmond J. Oathesa,b, Philip A. Cooka,d, Romain Duprata,b, Tyler M. Mooree, Maria A. Oquendob, Mary L. Phillipsf, Melvin McInnisg, Maurizio Favah, Madhukar H. Trivedii, Patrick McGrathj, Ramin Parseyk, Myrna M. Weissmanj, and Yvette I. Shelinea,b,d,l,1 aCenter for Neuromodulation in Depression and Stress, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; bDepartment of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; cDepartment of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; dDepartment of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; eBrain and Behavior Laboratory, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104; fDepartment of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA 15260; gDepartment of Psychiatry, University of Michigan School of Medicine, Ann Arbor, MI 48109; hDepartment of Psychiatry, Massachusetts General Hospital, Boston, MA 02114; iCenter for Depression Research and Clinical Care, Peter O'Donnell Institute, University of Texas Southwestern Medical Center, Dallas, TX 75390; jDepartment of Psychiatry, Columbia University College of Physicians & Surgeons, New York, NY 10032; -

Dissociations Between Microstructural and Functional Hierarchies Within Regions of Transmodal Cortex
bioRxiv preprint doi: https://doi.org/10.1101/488700; this version posted December 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. DISSOCIATIONS BETWEEN MICROSTRUCTURAL AND FUNCTIONAL HIERARCHIES WITHIN REGIONS OF TRANSMODAL CORTEX PAQUOLA C1, VOS DE WAEL R1, WAGSTYL K2, BETHLEHEM RAI3, HONG SJ1, SEIDLITZ J4,5, BULLMORE ET5, EVANS AC1,2, MISIC B1, MARGULIES DS6, SMALLWOOD J7, BERNHARDT BC1* 1 McConnell Brain Imaging Centre, Montreal Neurological Institute and Hospital, McGill University, Montreal, QC, Canada; 2 McGill Centre for Integrative Neuroscience, McGill University, Montreal, QC, Canada; 3 Autism Research Centre, Department of Psychiatry, University of Cambridge, England, United Kingdom; 4 Developmental Neurogenomics Unit, National Institute of Mental Health, Bethesda, MD 20892, USA; 5 Brain Mapping Unit, University of Cambridge, Department of Psychiatry, Cambridge CB2 0SZ, UK; 6 Frontlab, Institut du Cerveau et de la Moelle épinière, UPMC UMRS 1127, Inserm U 1127, CNRS UMR 7225, Paris, France; 7 York Neuroimaging Center, University of York, UK SUMMARY While the role of cortical microstructure in organising neural function is well established, it remains unclear how structural constraints can give rise to more flexible elements of cognition. While non- human primate research has demonstrated a close structure-function correspondence, the relationship between microstructure and function remains poorly understood in humans, in part because of the reliance on post mortem analyses which cannot be directly related to functional data. To overcome this barrier, we developed a novel approach to model the similarity of microstructural profiles sampled in the direction of cortical columns. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Neurogenetic Profiles Delineate Large-Scale Connectivity Dynamics
ARTICLE DOI: 10.1038/s41467-018-06346-3 OPEN Neurogenetic profiles delineate large-scale connectivity dynamics of the human brain Ibai Diez1,2 & Jorge Sepulcre1,3 Experimental and modeling work of neural activity has described recurrent and attractor dynamic patterns in cerebral microcircuits. However, it is still poorly understood whether similar dynamic principles exist or can be generalizable to the large-scale level. Here, we 1234567890():,; applied dynamic graph theory-based analyses to evaluate the dynamic streams of whole- brain functional connectivity over time across cognitive states. Dynamic connectivity in local networks is located in attentional areas during tasks and primary sensory areas during rest states, and dynamic connectivity in distributed networks converges in the default mode network (DMN) in both task and rest states. Importantly, we find that distinctive dynamic connectivity patterns are spatially associated with Allen Human Brain Atlas genetic tran- scription levels of synaptic long-term potentiation and long-term depression-related genes. Our findings support the neurobiological basis of large-scale attractor-like dynamics in the heteromodal cortex within the DMN, irrespective of cognitive state. 1 Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston 02114 MA, USA. 2 Neurotechnology Laboratory, Health Department, Tecnalia Research & Innovation, Derio 48160, Spain. 3 Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, -

Taste Quality Representation in the Human Brain
bioRxiv preprint doi: https://doi.org/10.1101/726711; this version posted August 6, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. bioRxiv (2019) Taste quality representation in the human brain Jason A. Avery?, Alexander G. Liu, John E. Ingeholm, Cameron D. Riddell, Stephen J. Gotts, and Alex Martin Laboratory of Brain and Cognition, National Institute of Mental Health, Bethesda, MD, United States 20892 Submitted Online August 5, 2019 SUMMARY In the mammalian brain, the insula is the primary cortical substrate involved in the percep- tion of taste. Recent imaging studies in rodents have identified a gustotopic organization in the insula, whereby distinct insula regions are selectively responsive to one of the five basic tastes. However, numerous studies in monkeys have reported that gustatory cortical neurons are broadly-tuned to multiple tastes, and tastes are not represented in discrete spatial locations. Neu- roimaging studies in humans have thus far been unable to discern between these two models, though this may be due to the relatively low spatial resolution employed in taste studies to date. In the present study, we examined the spatial representation of taste within the human brain us- ing ultra-high resolution functional magnetic resonance imaging (MRI) at high magnetic field strength (7-Tesla). During scanning, participants tasted sweet, salty, sour and tasteless liquids, delivered via a custom-built MRI-compatible tastant-delivery system. Our univariate analyses revealed that all tastes (vs. tasteless) activated primary taste cortex within the bilateral dorsal mid-insula, but no brain region exhibited a consistent preference for any individual taste. -

Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: a Data-Driven Multivoxel Pattern Analysis (MVPA)
Journal of Clinical Medicine Article Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: A Data-Driven Multivoxel Pattern Analysis (MVPA) Daniel R. Westfall 1 , Sheeba A. Anteraper 1,2,3, Laura Chaddock-Heyman 4, Eric S. Drollette 5, Lauren B. Raine 1 , Susan Whitfield-Gabrieli 1,2, Arthur F. Kramer 1,4 and Charles H. Hillman 1,6,* 1 Department of Psychology, Northeastern University, Boston, MA 02115, USA; [email protected] (D.R.W.); [email protected] (S.A.A.); [email protected] (L.B.R.); s.whitfi[email protected] (S.W.-G.); [email protected] (A.F.K.) 2 Northeastern University Biomedical Imaging Center, Northeastern University, Boston, MA 02115, USA 3 Alan and Lorraine Bressler Clinical and Research Program for Autism Spectrum Disorder, Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114, USA 4 Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA; [email protected] 5 Department of Kinesiology, University of North Carolina at Greensboro, Greensboro, NC 27402, USA; [email protected] 6 Department of Physical Therapy, Movement & Rehabilitation Sciences, Northeastern University, Boston, MA 02115, USA * Correspondence: [email protected] Received: 17 August 2020; Accepted: 30 September 2020; Published: 2 October 2020 Abstract: Scholastic performance is the key metric by which schools measure student’s academic success, and it is important to understand the neural-correlates associated with greater scholastic performance. This study examines resting-state functional connectivity (RsFc) associated with scholastic performance (reading and mathematics) in preadolescent children (7–9 years) using an unbiased whole-brain connectome-wide multi-voxel pattern analysis (MVPA). -

Functional Brain Connectivity at Rest Changes After Working Memory Training
r Human Brain Mapping 00:000–000 (2011) r Functional Brain Connectivity at Rest Changes After Working Memory Training Dietsje D. Jolles,1,2,3* Mark A. van Buchem,1,3 Eveline A. Crone,1,2 and Serge A.R.B. Rombouts1,2,3 1Leiden Institute for Brain and Cognition (LIBC), Leiden University, P.O. Box 9600, 2300 RC Leiden, The Netherlands 2Institute of Psychology, Leiden University, Wassenaarseweg 52, 2333 AK Leiden, The Netherlands 3Department of Radiology, Leiden University Medical Center, P.O. Box 9600, 2300 RC Leiden, The Netherlands r r Abstract: Networks of functional connectivity are highly consistent across participants, suggesting that functional connectivity is for a large part predetermined. However, several studies have shown that functional connectivity may change depending on instructions or previous experience. In the present study, we investigated whether 6 weeks of practice with a working memory task changes functional connectivity during a resting period preceding the task. We focused on two task-relevant networks, the frontoparietal network and the default network, using seed regions in the right middle frontal gyrus (MFG) and the medial prefrontal cortex (PFC), respectively. After practice, young adults showed increased functional connectivity between the right MFG and other regions of the frontoparietal net- work, including bilateral superior frontal gyrus, paracingulate gyrus, and anterior cingulate cortex. In addition, they showed reduced functional connectivity between the medial PFC and right posterior middle temporal gyrus. Moreover, a regression with performance changes revealed a positive relation between performance increases and changes of frontoparietal connectivity, and a negative relation between performance increases and changes of default network connectivity. -

Neuroimage: Clinical 15 (2017) 513–524
NeuroImage: Clinical 15 (2017) 513–524 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Disruption to control network function correlates with altered dynamic MARK connectivity in the wider autism spectrum ⁎ N. de Lacya,b, D. Dohertyb,c, B.H. Kingd, S. Rachakondae, V.D. Calhoune,f, a Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195, USA b Seattle Children's Research Institute, Center for Integrative Brain Research, Seattle, WA 98105, USA c Department of Pediatrics, Divisions of Developmental and Genetic Medicine, University of Washington, Seattle, WA 98195, USA d Department of Psychiatry, University of California San Francisco, San Francisco, CA 94143, USA e The Mind Research Network & LBERI, Albuquerque, NM 87106, USA f Department of Electrical and Computer Engineering, University of New Mexico, Albuquerque, NM 87131, USA ARTICLE INFO ABSTRACT Keywords: Autism is a common developmental condition with a wide, variable range of co-occurring neuropsychiatric Autism symptoms. Contrasting with most extant studies, we explored whole-brain functional organization at multiple Functional MRI levels simultaneously in a large subject group reflecting autism's clinical diversity, and present the first network- Dynamic connectivity based analysis of transient brain states, or dynamic connectivity, in autism. Disruption to inter-network and inter- Control networks system connectivity, rather than within individual networks, predominated. We identified coupling disruption in Task-positive the anterior-posterior default mode axis, and among specific control networks specialized for task start cues and Default-mode the maintenance of domain-independent task positive status, specifically between the right fronto-parietal and cingulo-opercular networks and default mode network subsystems.