Evolution of the Earliest Horses Driven by Climate Change in the Paleocene-Eocene Thermal Maximum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolution of Micro-Cursoriality in Mammals
© 2014. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2014) 217, 1316-1325 doi:10.1242/jeb.095737 RESEARCH ARTICLE The evolution of micro-cursoriality in mammals Barry G. Lovegrove* and Metobor O. Mowoe* ABSTRACT Perissodactyla) in response to the emergence of open landscapes and In this study we report on the evolution of micro-cursoriality, a unique grasslands following the Eocene Thermal Maximum (Janis, 1993; case of cursoriality in mammals smaller than 1 kg. We obtained new Janis and Wilhelm, 1993; Yuanqing et al., 2007; Jardine et al., 2012; running speed and limb morphology data for two species of elephant- Lovegrove, 2012b; Lovegrove and Mowoe, 2013). shrews (Elephantulus spp., Macroscelidae) from Namaqualand, Loosely defined, cursorial mammals are those that run fast. South Africa, which we compared with published data for other However, more explicit definitions of cursoriality remain obscure mammals. Elephantulus maximum running speeds were higher than because locomotor performance is influenced by multiple variables, those of most mammals smaller than 1 kg. Elephantulus also including behaviour, biomechanics, physiology and morphology possess exceptionally high metatarsal:femur ratios (1.07) that are (Taylor et al., 1970; Garland, 1983a; Garland, 1983b; Garland and typically associated with fast unguligrade cursors. Cursoriality evolved Janis, 1993; Stein and Casinos, 1997; Carrano, 1999). In an in the Artiodactyla, Perissodactyla and Carnivora coincident with evaluation of these definition problems, Carrano (Carrano, 1999) global cooling and the replacement of forests with open landscapes argued that ‘…morphology should remain the fundamental basis for in the Oligocene and Miocene. The majority of mammal species, making distinctions between locomotor performance…’. -

Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando
Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando To cite this version: Pablo Librado, Ludovic Orlando. Genomics and the Evolutionary History of Equids. Annual Review of Animal Biosciences, Annual Reviews, 2021, 9 (1), 10.1146/annurev-animal-061220-023118. hal- 03030307 HAL Id: hal-03030307 https://hal.archives-ouvertes.fr/hal-03030307 Submitted on 30 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Anim. Biosci. 2021. 9:X–X https://doi.org/10.1146/annurev-animal-061220-023118 Copyright © 2021 by Annual Reviews. All rights reserved Librado Orlando www.annualreviews.org Equid Genomics and Evolution Genomics and the Evolutionary History of Equids Pablo Librado and Ludovic Orlando Laboratoire d’Anthropobiologie Moléculaire et d’Imagerie de Synthèse, CNRS UMR 5288, Université Paul Sabatier, Toulouse 31000, France; email: [email protected] Keywords equid, horse, evolution, donkey, ancient DNA, population genomics Abstract The equid family contains only one single extant genus, Equus, including seven living species grouped into horses on the one hand and zebras and asses on the other. In contrast, the equine fossil record shows that an extraordinarily richer diversity existed in the past and provides multiple examples of a highly dynamic evolution punctuated by several waves of explosive radiations and extinctions, cross-continental migrations, and local adaptations. -

Proceedings of the United States National Museum
PALEOCENE PRIMATES OF THE FORT UNION, WITH DIS- CUSSION OF RELATIONSHIPS OF EOCENE PRIMATES. By James Williams Gidley, Assistant Curator, United States National Museum. INTRODUCTION. The first important contribution to the knowledge of Fort Union mammalian life was furnished by Dr. Earl Douglass and was based on a small lot of fragmentary material collected by him in the au- tumn of 1901 from a locality in Sweet Grass County, Montana, about 25 miles northeast of Bigtimber.* The fauna described by Douglass indicated a horizon about equivalent in age to the Torrejon of New Mexico, but the presence of unfamilar forms, suggesting a different faunal phase, was recognized. A few years later (1908 to 1911) this region was much more fully explored for fossil remains by parties of the United States Geological Survey and the United States National Museum. Working under the direction of Dr. T. W. Stanton, Mr. Albert C. Silberling, an ener- getic and successful collector, procured the first specimens in the winter and spring of 1908, continuing operations intermittently through the following years until the early spring of 1911. The present writer visited the field in 1908 and again in 1909, securing a considerable amount of good material. The net result of this com- bined field work is the splendid collection now in the National Museum, consisting of about 1,000 specimens, for the most part upper and lower jaw portions carrying teeth in varying numbers, but including also several characteristic foot and limb bones. Although nearly 10 years have passed since the last of this collec- tion was received, it was not until late in the summer of 1920 that the preparation of the material for study was completed. -

Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology
Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology by Paul David Polly B.A. (University of Texas at Austin) 1987 A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Paleontology in the GRADUATE DIVISION of the UNIVERSITY of CALIFORNIA at BERKELEY Committee in charge: Professor William A. Clemens, Chair Professor Kevin Padian Professor James L. Patton Professor F. Clark Howell 1993 Hyaenodontidae (Creodonta, Mammalia) and the Position of Systematics in Evolutionary Biology © 1993 by Paul David Polly To P. Reid Hamilton, in memory. iii TABLE OF CONTENTS Introduction ix Acknowledgments xi Chapter One--Revolution and Evolution in Taxonomy: Mammalian Classification Before and After Darwin 1 Introduction 2 The Beginning of Modern Taxonomy: Linnaeus and his Predecessors 5 Cuvier's Classification 10 Owen's Classification 18 Post-Darwinian Taxonomy: Revolution and Evolution in Classification 24 Kovalevskii's Classification 25 Huxley's Classification 28 Cope's Classification 33 Early 20th Century Taxonomy 42 Simpson and the Evolutionary Synthesis 46 A Box Model of Classification 48 The Content of Simpson's 1945 Classification 50 Conclusion 52 Acknowledgments 56 Bibliography 56 Figures 69 Chapter Two: Hyaenodontidae (Creodonta, Mammalia) from the Early Eocene Four Mile Fauna and Their Biostratigraphic Implications 78 Abstract 79 Introduction 79 Materials and Methods 80 iv Systematic Paleontology 80 The Four Mile Fauna and Wasatchian Biostratigraphic Zonation 84 Conclusion 86 Acknowledgments 86 Bibliography 86 Figures 87 Chapter Three: A New Genus Eurotherium (Creodonta, Mammalia) in Reference to Taxonomic Problems with Some Eocene Hyaenodontids from Eurasia (With B. Lange-Badré) 89 Résumé 90 Abstract 90 Version française abrégéé 90 Introduction 93 Acknowledgments 96 Bibliography 96 Table 3.1: Original and Current Usages of Genera and Species 99 Table 3.2: Species Currently Included in Genera Discussed in Text 101 Chapter Four: The skeleton of Gazinocyon vulpeculus n. -

Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming
Distribution and Stratigraphip Correlation of Upper:UB_ • Ju Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming U,S. GEOLOGICAL SURVEY PROFESS IONAL PAPER 1540 Cover. A member of the American Museum of Natural History 1896 expedition enter ing the badlands of the Willwood Formation on Dorsey Creek, Wyoming, near what is now U.S. Geological Survey fossil vertebrate locality D1691 (Wardel Reservoir quadran gle). View to the southwest. Photograph by Walter Granger, courtesy of the Department of Library Services, American Museum of Natural History, New York, negative no. 35957. DISTRIBUTION AND STRATIGRAPHIC CORRELATION OF UPPER PALEOCENE AND LOWER EOCENE FOSSIL MAMMAL AND PLANT LOCALITIES OF THE FORT UNION, WILLWOOD, AND TATMAN FORMATIONS, SOUTHERN BIGHORN BASIN, WYOMING Upper part of the Will wood Formation on East Ridge, Middle Fork of Fifteenmile Creek, southern Bighorn Basin, Wyoming. The Kirwin intrusive complex of the Absaroka Range is in the background. View to the west. Distribution and Stratigraphic Correlation of Upper Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Tatman Formations, Southern Bighorn Basin, Wyoming By Thomas M. Down, Kenneth D. Rose, Elwyn L. Simons, and Scott L. Wing U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1540 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsch, Acting Director For sale by U.S. Geological Survey, Map Distribution Box 25286, MS 306, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Description and Correlation of Eocene Rocks in Stratigraphie Reference Sections for the Green River and Washakie Basins, Southwest Wyoiming
Description and Correlation of Eocene Rocks in Stratigraphie Reference Sections for the Green River and Washakie Basins, Southwest Wyoiming U.S. GEOLOGICAL SURVEY PROFESSIONAE PAPER 1506-D Description and Correlation of Eocene Rocks in Stratigraphic Reference Sections for the Green River and Washakie Basins, Southwest Wyoming By HENRY W. ROEHLER GEOLOGY OF THE EOCENE WASATCH, GREEN RIVER, AND BRIDGER (WASHAKIE) FORMATIONS, GREATER GREEN RIVER BASIN, WYOMING, UTAH, AND COLORADO U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1506-D Includes analyses of Eocene rocks in the Washakie basin UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1992 U.S. DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government Library of Congress Cataloging in Publication Data Roehler, Henry W. Description and correlation of Eocene rocks in stratigraphic reference sections for the Green River and Washakie basins, Southwest Wyoming : includes analyses of Eocene rocks in the Washakie Basin / by Henry W. Roehler. p. cm. (Geology of the Eocene Wasatch, Green River and Bridger (Washakie) formations, greater Green River Basin, Wyoming, Utah, and Colorado) (U.S. Geological Survey professional paper ; 1506-D) Includes bibliographical references. Supt. of Docs, no.: I 19.16:1506-D 1. Geology, Stratigraphic Eocene. 2. Stratigraphic correlation Wyoming. 3. Geology Wyoming. I. Title. II. Series. III. Series: U.S. Geological Survey professional paper : 1506-D. QE692.2.R58 1992 551.7'84'09787 dc20 91-4442 CIP For sale by Book and Open-File Report Sales, U.S. -

Perissodactyla, Mammalia) from the Middle Eocene of Myanmar Un Nouveau Tapiromorphe Basal (Perissodactyla, Mammalia) De L’Eocène Moyen Du Myanmar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Geobios 39 (2006) 513–519 http://france.elsevier.com/direct/GEOBIO/ Original article A new basal tapiromorph (Perissodactyla, Mammalia) from the middle Eocene of Myanmar Un nouveau tapiromorphe basal (Perissodactyla, Mammalia) de l’Eocène moyen du Myanmar Grégoire Métais a, Aung Naing Soe b, Stéphane Ducrocq c,* a Carnegie Museum of Natural History, Section of Vertebrate Paleontology, 4400 Forbes Avenue, Pittsburgh, PA 15213, USA b Department of Geology, Yangon University, Yangon 11422, Myanmar c Laboratoire de Géobiologie, Biochronologie et Paléontologie Humaine, UMR 6046 CNRS, Faculté des Sciences de Poitiers, 40, avenue du recteur-Pineau, 86022 Poitiers cedex, France Received 29 September 2004; accepted 10 May 2005 Available online 20 March 2006 Abstract A new genus and species of tapiromorph, Skopaiolophus burmese nov. gen., nov. sp., is described from the middle Eocene Pondaung For- mation in central Myanmar. This small form displays a striking selenolophodont morphology associated with a mixture of primitive “condylar- thran” dental characters and derived tapiromorph features. Skopaiolophus is here tentatively referred to a group of Asian tapiromorphs unknown so far. The occurrence of such a form in Pondaung suggests that primitive tapiromorphs might have persisted in southeast Asia until the late middle Eocene while they became extinct elsewhere in both Eurasia and North America. © 2006 Elsevier SAS. All rights reserved. Résumé Un nouveau genre et une nouvelle espèce de tapiromorphe, Skopaiolophus burmese nov. gen. nov. sp., sont décrits dans la Formation de Pondaung d’âge fini-éocène moyen, au Myanmar. -

Resolving the Relationships of Paleocene Placental Mammals
Biol. Rev. (2015), pp. 000–000. 1 doi: 10.1111/brv.12242 Resolving the relationships of Paleocene placental mammals Thomas J. D. Halliday1,2,∗, Paul Upchurch1 and Anjali Goswami1,2 1Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, U.K. 2Department of Genetics, Evolution and Environment, University College London, Gower Street, London WC1E 6BT, U.K. ABSTRACT The ‘Age of Mammals’ began in the Paleocene epoch, the 10 million year interval immediately following the Cretaceous–Palaeogene mass extinction. The apparently rapid shift in mammalian ecomorphs from small, largely insectivorous forms to many small-to-large-bodied, diverse taxa has driven a hypothesis that the end-Cretaceous heralded an adaptive radiation in placental mammal evolution. However, the affinities of most Paleocene mammals have remained unresolved, despite significant advances in understanding the relationships of the extant orders, hindering efforts to reconstruct robustly the origin and early evolution of placental mammals. Here we present the largest cladistic analysis of Paleocene placentals to date, from a data matrix including 177 taxa (130 of which are Palaeogene) and 680 morphological characters. We improve the resolution of the relationships of several enigmatic Paleocene clades, including families of ‘condylarths’. Protungulatum is resolved as a stem eutherian, meaning that no crown-placental mammal unambiguously pre-dates the Cretaceous–Palaeogene boundary. Our results support an Atlantogenata–Boreoeutheria split at the root of crown Placentalia, the presence of phenacodontids as closest relatives of Perissodactyla, the validity of Euungulata, and the placement of Arctocyonidae close to Carnivora. Periptychidae and Pantodonta are resolved as sister taxa, Leptictida and Cimolestidae are found to be stem eutherians, and Hyopsodontidae is highly polyphyletic. -

Rapid and Early Post-Flood Mammalian Diversification Videncede in the Green River Formation
The Proceedings of the International Conference on Creationism Volume 6 Print Reference: Pages 449-457 Article 36 2008 Rapid and Early Post-Flood Mammalian Diversification videncedE in the Green River Formation John H. Whitmore Cedarville University Kurt P. Wise Southern Baptist Theological Seminary Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Whitmore, John H. and Wise, Kurt P. (2008) "Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation," The Proceedings of the International Conference on Creationism: Vol. 6 , Article 36. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol6/iss1/36 In A. A. Snelling (Ed.) (2008). Proceedings of the Sixth International Conference on Creationism (pp. 449–457). Pittsburgh, PA: Creation Science Fellowship and Dallas, TX: Institute for Creation Research. Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation John H. Whitmore, Ph.D., Cedarville University, 251 N. Main Street, Cedarville, OH 45314 Kurt P. Wise, Ph.D., Southern Baptist Theological Seminary, 2825 Lexington Road. -

Phylogeny of the Carnivoramorpha: the Impact of Postcranial Characters Michelle Spaulding a B D & John J
This article was downloaded by: [American Museum of Natural History] On: 01 February 2013, At: 10:50 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Systematic Palaeontology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tjsp20 Phylogeny of the Carnivoramorpha: The impact of postcranial characters Michelle Spaulding a b d & John J. Flynn b c a Department of Earth & Environmental Sciences, Columbia University, New York, NY, 10027, USA b Division of Paleontology, American Museum of Natural History, Central Park West at 79th Street, New York, NY, 10024, USA c Richard Gilder Graduate School, American Museum of Natural History, Central Park West at 79th Street, New York, NY, 10024, USA d Section of Mammals, Carnegie Museum of Natural History, 5800 Baum Boulevard, Pittsburgh, Pennsylvania, 15206, USA Version of record first published: 25 Apr 2012. To cite this article: Michelle Spaulding & John J. Flynn (2012): Phylogeny of the Carnivoramorpha: The impact of postcranial characters, Journal of Systematic Palaeontology, 10:4, 653-677 To link to this article: http://dx.doi.org/10.1080/14772019.2011.630681 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -
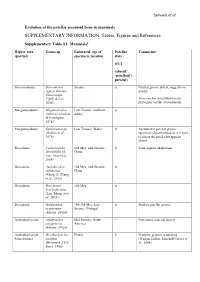
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp.