Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nucleotide Metabolism
NUCLEOTIDE METABOLISM General Overview • Structure of Nucleotides Pentoses Purines and Pyrimidines Nucleosides Nucleotides • De Novo Purine Nucleotide Synthesis PRPP synthesis 5-Phosphoribosylamine synthesis IMP synthesis Inhibitors of purine synthesis Synthesis of AMP and GMP from IMP Synthesis of NDP and NTP from NMP • Salvage pathways for purines • Degradation of purine nucleotides • Pyrimidine synthesis Carbamoyl phosphate synthesisOrotik asit sentezi • Pirimidin nükleotitlerinin yıkımı • Ribonükleotitlerin deoksiribonükleotitlere dönüşümü Basic functions of nucleotides • They are precursors of DNA and RNA. • They are the sources of activated intermediates in lipid and protein synthesis (UDP-glucose→glycogen, S-adenosylmathionine as methyl donor) • They are structural components of coenzymes (NAD(P)+, FAD, and CoA). • They act as second messengers (cAMP, cGMP). • They play important role in carrying energy (ATP, etc). • They play regulatory roles in various pathways by activating or inhibiting key enzymes. Structures of Nucleotides • Nucleotides are composed of 1) A pentose monosaccharide (ribose or deoxyribose) 2) A nitrogenous base (purine or pyrimidine) 3) One, two or three phosphate groups. Pentoses 1.Ribose 2.Deoxyribose •Deoxyribonucleotides contain deoxyribose, while ribonucleotides contain ribose. •Ribose is produced in the pentose phosphate pathway. Ribonucleotide reductase converts ribonucleoside diphosphate deoxyribonucleotide. Nucleotide structure-Base 1.Purine 2.Pyrimidine •Adenine and guanine, which take part in the structure -

Effects of Allopurinol and Oxipurinol on Purine Synthesis in Cultured Human Cells
Effects of allopurinol and oxipurinol on purine synthesis in cultured human cells William N. Kelley, James B. Wyngaarden J Clin Invest. 1970;49(3):602-609. https://doi.org/10.1172/JCI106271. Research Article In the present study we have examined the effects of allopurinol and oxipurinol on thed e novo synthesis of purines in cultured human fibroblasts. Allopurinol inhibits de novo purine synthesis in the absence of xanthine oxidase. Inhibition at lower concentrations of the drug requires the presence of hypoxanthine-guanine phosphoribosyltransferase as it does in vivo. Although this suggests that the inhibitory effect of allopurinol at least at the lower concentrations tested is a consequence of its conversion to the ribonucleotide form in human cells, the nucleotide derivative could not be demonstrated. Several possible indirect consequences of such a conversion were also sought. There was no evidence that allopurinol was further utilized in the synthesis of nucleic acids in these cultured human cells and no effect of either allopurinol or oxipurinol on the long-term survival of human cells in vitro could be demonstrated. At higher concentrations, both allopurinol and oxipurinol inhibit the early steps ofd e novo purine synthesis in the absence of either xanthine oxidase or hypoxanthine-guanine phosphoribosyltransferase. This indicates that at higher drug concentrations, inhibition is occurring by some mechanism other than those previously postulated. Find the latest version: https://jci.me/106271/pdf Effects of Allopurinol and Oxipurinol on Purine Synthesis in Cultured Human Cells WILLIAM N. KELLEY and JAMES B. WYNGAARDEN From the Division of Metabolic and Genetic Diseases, Departments of Medicine and Biochemistry, Duke University Medical Center, Durham, North Carolina 27706 A B S TR A C T In the present study we have examined the de novo synthesis of purines in many patients. -

Purine and Pyrimidine Biosynthesis
Biosynthesis of Purine & Pyrimidi ne Introduction Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined together to form macromolecules. This process often consists of metabolic pathways. The purines are built upon a pre-existing ribose 5- phosphate. Liver is the major site for purine nucleotide synthesis. Erythrocytes, polymorphonuclear leukocytes & brain cannot produce purines. Pathways • There are Two pathways for the synthesis of nucleotides: 1. De-novo synthesis: Biochemical pathway where nucleotides are synthesized from new simple precursor molecules 2. Salvage pathway: Used to recover bases and nucleotides formed during the degradation of RNA and DNA. Step involved in purine biosynthesis (Adenine & Guanine) • Ribose-5-phosphate, of carbohydrate metabolism is the starting material for purine nucleotide synthesis. • It reacts with ATP to form phosphoribosyl pyrophosphate (PRPP). • Glutamine transfers its amide nitrogen to PRPP to replace pyrophosphate & produce 5- phosphoribosylamine. Catalysed by PRPP glutamyl amidotransferase. • This reaction is the committed. • Phosphoribosylamine reacts with glycine in the presence of ATP to form glycinamide ribosyl 5- phosphate or glycinamide ribotide (GAR).Catalyzed by synthetase. • N10-Formyl tetrahydrofolate donates the formyl group & the product formed is formylglycinamide ribosyl 5-phosphate. Catalyzed by formyltransferase. • Glutamine transfers the second amido amino group to produce formylglycinamidine ribosyl 5- phosphate. Catalyzed by synthetase. • The imidazole ring of the purine is closed in an ATP dependent reaction to yield 5- aminoimidazole ribosyl 5-phosphate. Catalyzed by synthetase. • Incorporation of CO2 (carboxylation) occurs to yield aminoimidazole carboxylate ribosyl 5- phosphate. -

Induced Alterations in the Urine Metabolome in Cardiac Surgery
www.nature.com/scientificreports OPEN Bretschneider solution- induced alterations in the urine metabolome in cardiac surgery Received: 16 August 2018 Accepted: 1 November 2018 patients Published: xx xx xxxx Cheng-Chia Lee1,3, Ya-Ju Hsieh 2, Shao-Wei Chen3,4, Shu-Hsuan Fu2, Chia-Wei Hsu 2, Chih-Ching Wu 2,5,6, Wei Han7, Yunong Li7, Tao Huan7, Yu-Sun Chang2,6,8, Jau-Song Yu2,9,10, Liang Li7, Chih-Hsiang Chang1,3 & Yi-Ting Chen 1,2,11,12 The development of Bretschneider’s histidine-tryptophan-ketoglutarate (HTK) cardioplegia solution represented a major advancement in cardiac surgery, ofering signifcant myocardial protection. However, metabolic changes induced by this additive in the whole body have not been systematically investigated. Using an untargeted mass spectrometry-based method to deeply explore the urine metabolome, we sought to provide a holistic and systematic view of metabolic perturbations occurred in patients receiving HTK. Prospective urine samples were collected from 100 patients who had undergone cardiac surgery, and metabolomic changes were profled using a high-performance chemical isotope labeling liquid chromatography-mass spectrometry (LC-MS) method. A total of 14,642 peak pairs or metabolites were quantifed using diferential 13C-/12C-dansyl labeling LC-MS, which targets the amine/phenol submetabolome from urine specimens. We identifed 223 metabolites that showed signifcant concentration change (fold change > 5) and assembled several potential metabolic pathway maps derived from these dysregulated metabolites. Our data indicated upregulated histidine metabolism with subsequently increased glutamine/glutamate metabolism, altered purine and pyrimidine metabolism, and enhanced vitamin B6 metabolism in patients receiving HTK. -

NUCLEOTIDE METABOLISM Mark Rush
NUCLEOTIDE METABOLISM Mark Rush Nucleotides serve various metabolic functions. For example, they are: • Substrates (building blocks) for nucleic acid biosynthesis and repair, • The main storage form of “high energy phosphate”, • Components of many “so-called” co-enzymes (NAD, NADP, FAD, CoA), • Components of many activated metabolic intermediates (such as UDPG, SAM), • Major allosteric effectors (such as AMP, ADP, ATP, GTP), • Major second messengers (such as 3',5' cAMP), and • Precursors for the biosynthesis of a variety of important compounds (such as biopterin and histidine). Nucleotide biochemistry can be treated both as an aspect of nitrogen metabolism, along with such compounds as amino acids and porphyrins, and as an aspect of nucleic acid metabolism. When the focus is on the biosynthesis and degradation of nucleotides, in other words on their turnover, the treatment is similar to that of other nitrogenous compounds. When the focus is on the role of nucleotides in overall nucleic acid metabolism, the treatment is included in molecular biology. Both aspects will be considered here with the major emphasis directed toward relating defects in nucleotide turnover to either metabolic diseases or chemotherapy. I. Nomenclature (pages 11 and 12). II. Overall metabolic pathways (page 4). 1) PRPP synthetase. 2) Nucleoside phosphorylases. 3) Phosphoribosyl transferases. nucleotides - 1 III. Biosynthesis of purines and pyrimidines (pages 5, 6, 8). A minimum amount of time will be spent discussing these pathways in lecture. Please examine them carefully in the text and note that: 1) Purine synthesis begins at the nucleotide level, while pyrimidine synthesis does not. 2) Both syntheses are regulated at their committed steps. -

Comparing Meiothermus Ruber and Myxococcus Xanthus in the Purine Metabolism Pathway Linnea J
Augustana College Augustana Digital Commons Meiothermus ruber Genome Analysis Project Biology 2-2016 Comparing Meiothermus ruber and Myxococcus xanthus in the Purine Metabolism Pathway Linnea J. Ritchie Augustana College - Rock Island Dr. Lori Scott Augustana College, Rock Island Illinois Follow this and additional works at: http://digitalcommons.augustana.edu/biolmruber Part of the Bioinformatics Commons, Biology Commons, Genetics Commons, Genomics Commons, Molecular Biology Commons, and the Molecular Genetics Commons Recommended Citation Ritchie, Linnea J. and Scott, Dr. Lori. "Comparing Meiothermus ruber and Myxococcus xanthus in the Purine Metabolism Pathway" (2016). Meiothermus ruber Genome Analysis Project. http://digitalcommons.augustana.edu/biolmruber/7 This Student Paper is brought to you for free and open access by the Biology at Augustana Digital Commons. It has been accepted for inclusion in Meiothermus ruber Genome Analysis Project by an authorized administrator of Augustana Digital Commons. For more information, please contact [email protected]. Comparing Meiothermus ruber and Myxococcus xanthus in the Purine Metabolism Pathway Linnea Ritchie Bio-375 Molecular Genetics (Dr. Lori Scott) Background The purine metabolism pathway is an essential part of an organism’s ability to make nucleotides. It is through this pathway that adenine and guanine are made, these molecules later become the bases of nucleotides, which are a key component in DNA (Westby 1974). There are two different routes for purine synthesis: the de novo pathway and the salvage pathway (Berg 2002). During the de novo pathway the purine molecules are essentially built from scratch. While this route uses comparatively simple molecules and amino acids there is a high energy requirement which is why at times the salvage pathway is used instead. -

A Rare Bacterial RNA Motif Is Implicated in the Regulation of the Purf Gene Whose Encoded Enzyme Synthesizes Phosphoribosylamine
Downloaded from rnajournal.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press RNA (Report) RNA/2020/077313 Revised A rare bacterial RNA motif is implicated in the regulation of the purF gene whose encoded enzyme synthesizes phosphoribosylamine Sarah N. Malkowski,1 Ruben M. Atilho,2 Etienne B. Greenlee,3 Christina E. Weinberg,3,5 Ronald R. Breaker2,3,4 1Department of Chemistry, Yale University, New Haven, Connecticut 06520-8103, USA 2Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520-8103, USA 3Department of Molecular, Cellular and Developmental Biology, Yale University, New Haven, Connecticut 06520-8103, USA 4Howard Hughes Medical Institute, Yale University, New Haven, CT 06520-8103, USA 5Current address: Institute for Biochemistry, Leipzig University, Brüderstraße 34, 04103 Leipzig, Germany Corresponding author: Ronald R. Breaker, [email protected]. KEYWORDS: aptamer; gene control; noncoding RNA; PRA; purine; ribose Downloaded from rnajournal.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Malkowski et al. and Breaker Candidate PRA Regulatory RNA ABSTRACT The Fibro-purF motif is a putative structured noncoding RNA domain that was discovered previously in species of Fibrobacter by employing comparative sequence analysis methods. An updated bioinformatics search yielded a total of only 30 unique-sequence representatives, exclusively found upstream of the purF gene that codes for the enzyme amidophosphoribosyltransferase. This enzyme synthesizes the compound 5-phospho-D- ribosylamine (PRA), which is the first committed step in purine biosynthesis. The consensus model for Fibro-purF motif RNAs includes a predicted three-stem junction that carries numerous conserved nucleotide positions within the regions joining the stems. -
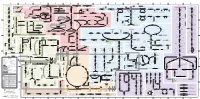
Fullsubwaymap221.Pdf
A B C D E F G H I J K L M Aldose reductase Sorbitol dehydrogenase Cholesterol synthesis Glycogen and galactose metabolism (branch point only) (debranching) glucose sorbitol fructose Aromatic amino acid metabolism Purine synthesis Pyrimidine synthesis glycogen (n+1) phenylacetate triiodothyronine (T3) dopaquinone melanin + + thyroxine (T4) (multiple steps) (many cycles) P 4' NADPH NADP NAD NADH acetyl-CoA x2 i Debranching enzyme 3' - α-1,4 bonds Aromatic amino acid 5-phosphoribosyl pyrophosphate (PRPP) HCO B 2' P 3 (branching) Glycogen 6 i (multiple steps) O H O (multiple steps) Tyrosinase O H O 1' 2 2 2 2 B 1 2 3 4 5 6 7 8 9 10 Pentose phosphate pathway Phenylalanine Tyrosine decarboxylase 6 Dopamine B Thiolase phosphorylase -5 -4 -3 -2 -1 0 1 2 3 4 Transaminase 6 glutamine 2 ATP (many cycles) Glycogen Glucose-6-phosphatase Dehydrogenase hydroxylase hydroxylase (DOPA decarboxylase) β-hydroxylase Methyltransferase 10 UDP 4:4 Transferase ATP glutathione (reduced) H O phenyllactate phenylpyruvate phenylalanine tyrosine dihydroxy- dopamine Glutamine PRPP CoA synthase Hexokinase or (in endoplasmic reticulum) 2 2 norepinephrine epinephrine glutamine Carbamoyl 9 1' phenylanine amidotransferase (GPAT) glutamate glycogen (n) Glutathione reductase Glutathione peroxidase + phosphate glycogen (n) -5 -4 -3 -2 -1 0 1 2 3 4 2' 3' 4' glucokinase 8 NADP NADPH glutamate α-ketoglutarate CO2 O H O Glycosyl (4:6) UDP-glucose ADP (L-DOPA) 2 2 synthetase II glutathione (oxidized) 2 H O α-ketoglutarate PPi glutamate acetoacetyl-CoA 7 1 Glucose -1,6-Glucosidase -

Purine Metabolism in Acholeplasma Laidlawii B: Novel Ppi- Dependent Nucleoside Kinase Activity VICTOR V
JOURNAL OF BACTERIOLOGY, July 1984, p. 265-270 Vol. 159, No. 1 0021-9193/84/070265-06$02.00/0 Copyright ©3 1984, American Society for Microbiology Purine Metabolism in Acholeplasma laidlawii B: Novel PPi- Dependent Nucleoside Kinase Activity VICTOR V. TRYON AND DENNIS POLLACK* Department of Medical Microbiology and Immunology, The Ohio State University, Columbuts, Ohio 43210 Received 21 February 1984/Accepted 12 April 1984 Acholeplasma Iaidlawii B-PG9 was examined for 16 cytoplasmic enzymes with activity for purine salvage and interconversion. Phosphoribosyltransferase activities for adenine, guanine, xanthine, and hypoxanthine were shown. Adenine, guanine, xanthine, and hypoxanthine were ribosylated to their nucleoside. Adenosine, inosine, xanthosine, and guanosine were converted to their base. No ATP-dependent phosphorylation of nucleosides to mononucleotides was found. However, PPi-dependent phosphorylation of adenosine, inosine, and guanosine to AMP, inosine monophosphate, and GMP, respectively, was detected. Nucleotidase activity for AMP, inosine monophosphate, xanthosine monophosphate, and GMP was also found. Interconversion of GMP to AMP was detected. Enzyme activities for the interconversion of AMP to GMP were not detected. Therefore, A. laidlawii B-PG9 cannot synthesize guanylates from adenylates or inosinates. De novo synthesis of purines was not detected. This study demonstrates that A. Iaidlawii B-PG9 has the enzyme activities for the salvage and limited interconversion of purines and, except for purine nucleoside kinase activity, is similar to Mycoplasma mycoides subsp. mycoides. This is the first report of a PPi-dependent nucleoside kinase activity in any organism. The only members of the class Mollicutes for which the phosphate ([8-14C]XMP), 56 mCi/mmol. [8-14C]inosine 5'- pathways of purine salvage and interconversion have been monophosphate ([8-14C]IMP) (59 mCi/mmol) was purchased comprehensively studied and described is Mycoplasma my- from Amersham Corp. -

Taxonomic Variations in the Gut Microbiome of Gout Patients With
Méndez‑Salazar et al. Mol Med (2021) 27:50 https://doi.org/10.1186/s10020‑021‑00311‑5 Molecular Medicine RESEARCH ARTICLE Open Access Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism Eder Orlando Méndez‑Salazar1,2†, Janitzia Vázquez‑Mellado3, Carlos S. Casimiro‑Soriguer4,5, Joaquin Dopazo4,5,6,7, Cankut Çubuk8, Yessica Zamudio‑Cuevas9, Adriana Francisco‑Balderas9, Karina Martínez‑Flores9, Javier Fernández‑Torres9, Carlos Lozada‑Pérez10, Carlos Pineda11, Austreberto Sánchez‑González12, Luis H. Silveira13, Ana I. Burguete‑García14, Citlalli Orbe‑Orihuela14, Alfredo Lagunas‑Martínez14, Alonso Vazquez‑Gomez15, Alberto López‑Reyes16, Berenice Palacios‑González1* and Gabriela Angélica Martínez‑Nava9† Abstract Objective: To evaluate the taxonomic composition of the gut microbiome in gout patients with and without tophi formation, and predict bacterial functions that might have an impact on urate metabolism. Methods: Hypervariable V3–V4 regions of the bacterial 16S rRNA gene from fecal samples of gout patients with and without tophi (n 33 and n 25, respectively) were sequenced and compared to fecal samples from 53 healthy controls. We explored= predictive =functional profles using bioinformatics in order to identify diferences in taxonomy and metabolic pathways. Results: We identifed a microbiome characterized by the lowest richness and a higher abundance of Phascolarc- tobacterium, Bacteroides, Akkermansia, and Ruminococcus_gnavus_group genera in patients with gout without tophi when compared to controls. The Proteobacteria phylum and the Escherichia-Shigella genus were more abundant in patients with tophaceous gout than in controls. Fold change analysis detected nine genera enriched in healthy controls compared to gout groups (Bifdobacterium, Butyricicoccus, Oscillobacter, Ruminococcaceae_UCG_010, Lach- nospiraceae_ND2007_group, Haemophilus, Ruminococcus_1, Clostridium_sensu_stricto_1, and Ruminococcaceae_ UGC_013). -

Nucleotides, Nucleic Acids: General Information About Structure, Functions and Metabolism
MINISTRY OF HEALTH OF UKRAINE ZAPORIZHZHIA STATE MEDICAL UNIVERSITY Biological Chemistry Department Nucleotides, Nucleic acids: General Information about Structure, Functions and Metabolism A manual for independent work at home and in class for students of second year study of international faculty Speciality “Medicine” Zaporizhzhia, 2016 1 UDC 577.1(075.8) BBC 28.902я73 N92 The manual was approved on the Central Methodological Council of ZSMU on «____» _______________2016, the protocol №________ Reviewers: Prykhodko O.B., Head of Medical Biology, Parasitology and Genetics Department of Zaporizhzhia State Medical University, doctor of biological science Voskoboynik O.Yu., associate professor of Organic and Bioorganic Chemistry Department of Zaporizhzhia State Medical University, PhD Editors: Dr. Hab., professor Aleksandrova K. V. PhD, assoc. professor Ivanchenko D. G. PhD, assoc. professor Krisanova N. V. Nucleotides, Nucleic acids : General Information about Structure, Functions and Metabolism : a manual for independent work at home and in class for students of second year study of international faculty, speciality ―Medicine‖/ ed. : K. V. Aleksandrova, D. G. Ivanchenko, N. V. Krisanova. – Zaporizhzhia : ZSMU, 2016.- 84 p. This manual is recommended to use for students of International Faculty (the second year of study) for independent work at home and in class. Нуклеотиди, нуклеїнові кислоти : загальне уявлення про структуру, функції та метаболізм : навч. посіб. для самостійної аудиторної та позааудиторної роботи студентів 2 курсу міжнар. ф-ту, спеціальність «Медицина» / ред.. : К. В. Александрова, Д. Г. Іванченко, Н. В. Крісанова. - Запоріжжя : ЗДМУ, 2016. – 84 с. UDC 577.1(075.8) BBC 28.902я73 ©Aleksandrova K.V., IvanchenkoD.G., Krisanova N.V., 2016 ©Zaporizhzhia State Medical University, 2016 2 INTRODUCTION A study of questions for this manual is the basis for learning of all metabolic pathways for nucleotides and nucleic acids. -

1 Synthesis of C-2 and C-6 Functionalized
1 SYNTHESIS OF C-2 AND C-6 FUNCTIONALIZED RIBOFURANOSYLPURINE ANALOGUES AS POTENTIAL ANTIVIRAL AGENTS TARGETING INHIBITION OF INOSINE MONOPHOSPHATE DEHYDROGENASE. by Eric Osei-Tutu Bonsu (Under the Direction of Vasu Nair) ABSTRACT IMPDH is a key enzyme in the de novo biosynthesis of purine nucleotides. It catalyzes the conversion of inosine monophosphate (IMP) to xanthosine monophosphate (XMP) using NAD as a cofactor. XMP is successively converted to deoxyguanosine triphosphate (substrate for DNA synthesis) by GMP synthetase, phosphorylating enzymes and ribonucleotide reductase. IMPDH exists in two isoforms, type I and type II. These isoforms have the same size and share 84% homology. The type I isoform is expressed in both normal and rapidly proliferating cells, whereas type II is preferentially upregulated in proliferating cells. Inhibition of IMPDH has anticancer, antiviral, antibacterial and immunosuppressive effects. Three inhibitors of IMPDH are currently in clinical use: ribavirin (a broad spectrum antiviral), mizoribine (immunosuppressant used in Japan) and mycophenolic mofetil (prodrug of mycophenolic acid, US approved immunosuppressant). None of these inhibitors possess significant selectivity against the type II isoform over the type I, hence there are severe side effects. The quest for specific isozyme inhibitors led to the discovery of the potential antiviral activity of certain C-2 functionalized hypoxanthine and C-2, C-6 modified purine systems against HSV1, HSV2, VV, VSV and RSV. 2 Nair and coworkers have synthesized similar congeners, including 2-vinylinosine (broad spectrum antiviral), which is active due to its C-2 vinyl moiety acting as a Michael Acceptor. This dissertation elucidates the design and synthesis of new Michael Acceptor-type nucleosides.