Oibijf? 1Sigo1offl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHITE RIVER NATIONAL FOREST Adam Mountain (8,200 Acres)
WHITE RIVER NATIONAL FOREST Adam Mountain (8,200 acres) ........................................................................................................ 3 Ashcroft (900 acres) ........................................................................................................................ 4 Assignation Ridge (13,300 acres) ................................................................................................... 4 Baldy Mountain (6,100 acres) ......................................................................................................... 6 Basalt Mountain A (13,900 acres) .................................................................................................. 6 Basalt Mountain (7,400 acres) ........................................................................................................ 7 Berry Creek (8,600 acres) ............................................................................................................... 8 Big Ridge to South Fork A (35,400 acres) and Big Ridge to South Fork B (6,000 acres) ............. 9 Black Lake East (800 acres) and Black Lake West (900 acres) ................................................... 11 Blair Mountain (500 acres) ........................................................................................................... 12 Boulder (1,300 acres) .................................................................................................................... 13 Budges (1,000 acres) .................................................................................................................... -
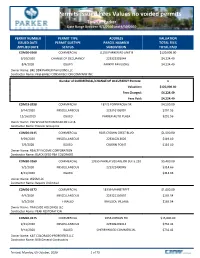
Permits Issued Fees Values No Voided Permits Town of Parker Date Range Between 9/1/2020 and 9/30/2020
Permits Issued Fees Values no voided permits Town of Parker Date Range Between 9/1/2020 and 9/30/2020 PERMIT NUMBER PERMIT TYPE ADDRESS VALUATION ISSUED DATE PERMIT SUBTYPE PARCEL NUMBER TOTAL FEES APPLIED DATE STATUS SUBDIVISION TOTAL PAID COM20-0160 COMMERCIAL 11153 PARKER RD UNIT B $120,000.00 9/10/2020 CHANGE OF OCCUPANCY 223322301044 $4,224.49 8/4/2020 ISSUED PARKER PAVILIONS $4,224.49 Owner Name: BRE DDR PARKER PAVILIONS LLC Contractor Name: PREFERRED CONSTRUCTION COMPANY INC Number of COMMERCIAL/CHANGE OF OCCUPANCY Permits: 1 Valuation: $120,000.00 Fees Charged: $4,224.49 Fees Paid: $4,224.49 COM19-0258 COMMERCIAL 18225 PONDEROSA DR $4,500.00 9/14/2020 MISCELLANEOUS 223316106001 $291.56 12/16/2019 ISSUED PARKER AUTO PLAZA $291.56 Owner Name: 736 W CASTLETON ROAD #C LLC & Contractor Name: Pioneer Group Inc COM20-0145 COMMERCIAL 9205 CROWN CREST BLVD $1,500.00 9/29/2020 MISCELLANEOUS 223310213026 $119.10 7/9/2020 ISSUED CROWN POINT $119.10 Owner Name: REALTY INCOME CORPORATION Contractor Name: BLACK EYED PEA COLORADO COM20-0169 COMMERCIAL 19555 PARKER SQUARE DR SUITE 210 $5,400.00 9/2/2020 MISCELLANEOUS 223322400045 $314.66 8/31/2020 ISSUED $314.66 Owner Name: W2SM LLC Contractor Name: Repairs Unlimited COM20-0172 COMMERCIAL 18139 MAINSTREET $1,000.00 9/4/2020 MISCELLANEOUS 223321110007 $165.54 9/2/2020 FINALED TRAILSIDE VILLAGE $165.54 Owner Name: TRAILSIDE HOLDINGS LLC Contractor Name: PEAK RESTORATION COM20-0175 COMMERCIAL 9256 JORDAN RD $15,000.00 9/21/2020 MISCELLANEOUS 223309201014 $732.42 9/14/2020 ISSUED CHERRYWOOD COMMERCIAL -

Summits on the Air – ARM for USA - Colorado (WØC)
Summits on the Air – ARM for USA - Colorado (WØC) Summits on the Air USA - Colorado (WØC) Association Reference Manual Document Reference S46.1 Issue number 3.2 Date of issue 15-June-2021 Participation start date 01-May-2010 Authorised Date: 15-June-2021 obo SOTA Management Team Association Manager Matt Schnizer KØMOS Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Page 1 of 11 Document S46.1 V3.2 Summits on the Air – ARM for USA - Colorado (WØC) Change Control Date Version Details 01-May-10 1.0 First formal issue of this document 01-Aug-11 2.0 Updated Version including all qualified CO Peaks, North Dakota, and South Dakota Peaks 01-Dec-11 2.1 Corrections to document for consistency between sections. 31-Mar-14 2.2 Convert WØ to WØC for Colorado only Association. Remove South Dakota and North Dakota Regions. Minor grammatical changes. Clarification of SOTA Rule 3.7.3 “Final Access”. Matt Schnizer K0MOS becomes the new W0C Association Manager. 04/30/16 2.3 Updated Disclaimer Updated 2.0 Program Derivation: Changed prominence from 500 ft to 150m (492 ft) Updated 3.0 General information: Added valid FCC license Corrected conversion factor (ft to m) and recalculated all summits 1-Apr-2017 3.0 Acquired new Summit List from ListsofJohn.com: 64 new summits (37 for P500 ft to P150 m change and 27 new) and 3 deletes due to prom corrections. -

Dillon Ranger District Permitted Outfitter Guides 2013 the Affable
Dillon Ranger District Permitted Outfitter Guides 2013 R2-WRNF-DRD COMPANY NAME PHONE WEB ADDRESS ACTIVITIES PERMITTED AREAS The Affable Angler Corporation, dba The Mountain Angler, 970-453-4665 www.mountainangler.com Fishing Blue River, Middle Fork Swan, South Fork Swan Ltd. Mountaineering, Rock-Climbing, Tenmile Canyon, Tenmile Range, Mount Royal, Swan Mountain Climbing Area, Cold Springs, Gold Hill, Miners Creek, Little French Gulch, Bald Mtn, 970-468-8049 www.rockymountainguides.com Ice-Climbing, Avlanche classes, Alpine Productions, LLC, dba Rocky Mountaineering Guides Lenawee Mtn, Uneva Peak, Quandary Peak, Francies & Janet's cabin, Hut trips Loveland Pass, Avalanche classes, Buffalo Mtn, Baldy & Mt. Guyot America's Adventure (AAVE Teen Adventures) 303-526-0806 www.aave.com Backpacking Gore Range Arkansas Valley Adventures 719-486-2187 www.coloradorafting.net Rafting Blue River, Dowd Chutes Avalanche & Backcountry Francies and Janets Cabins, Section House, Avalanche classes, Loveland Backcountry Babes, LLC 970 830-9975 www.backcountrybabes.net Education Pass Peru Creek, Boreas Pass, Sts. John, Lenawee, Chihuahua Gulch, Greys & Hiking, Biking, Snowhoeing, XC Torreys, Hunki Dori, Webster Pass, Morgan Gulch, Swan forks, Bakers Tank, Bodhi Enterprises Inc., dba Colorado Bike and Ski Tours 970-668-8900 www.cbstadventures.com Skiing, Rock Climbing, Mohawk Lakes, Mayflower Gulch, Peaks, Gold Hill, Mt. Royal, Miners Creek, Treking/Orienteering Wheeler CO Trail, Crystal Lakes, Quandary, Montezuma, Straight Creek, Peak 1, Hunting (GMU 37 & 371) / Horse Breckenridge Adventure Tours, dba Gore Range Outfitters 970-668-4485 www.gorerangeoutfitters.com Ptarmigan Wilderness, Eagles Nest Wilderness Trail Rides Boreas Pass, Swan Mtn, Vail Pass bike path, Peak 9, Keystone Bike path, Frisco-Breck bike path, Hoosier Pass, Mohawk Lakes, N. -

Colorado Rock Glacier Inventory: Active, Inactive and Relict Rock Glaciers
Colorado Rock Glacier Inventory: Active, Inactive and Relict Rock Glaciers By Abby McCarthy Research Experience for Undergraduates Program Portland State University Department of Geology Dr. Andrew Fountain (P.I.) Allison Trcka (Mentor) Photo: National Park Service Introduction / Method / Classification / Results Rock Glaciers Mass of rock, sediment and perennial ice, all flowing downslope Periglacial ● Methods of formation: Origin ○ Periglacial: when ice fills the space between rock debris A ○ Glacial: ice-core glacier is covered in rock debris ○ Combination of periglacial and Glacial glacial Origin ● Supply of rock debris and snow is important B Figure 1: Cross-sections of the formation of a rock glacier. Diagram: courtesy of Andrew Fountain Introduction / Method / Classification / Results Significance ● More resilient against climate change than other glaciers because debris insulates ice ● Fresh water source for ecosystems, agriculture and human use ● Cool air creates microclimates for plants and animals Figure 2: Pikas are a kind of animal that benefits from rock glaciers as a habitat. Photo: Dulude-De Broin Introduction / Method / Classification / Results Previous Inventories ● Johnson (2018) provides a template for locating rock glaciers nationally ● Janke (2007) classified rock glaciers around Rocky Mountain National Park in Colorado Issue that this study is addressing: ● Inconsistent classification of rock glaciers among studies Figure 3: Each rock glacier in the ● Non-rock-glacier features are wronlgy being Johnson (2018) inventory of the western U.S. is marked with a pink triangle. classified into rock glaciers Diagram: courtesy of Andrew Fountain Introduction / Method / Classification / Results What I did Located rock glaciers using Google Earth Pro Categorized rock glaciers into one of three categories, also marking features similar to rock glaciers Outlined rock glaciers on ArcMap Repeated this process for more than two thousand rock glaciers and features! Figure 4: The study area in Colorado is outlined in pink. -

L&I Contractor Authorized Signer Data
L&I Contractor Authorized Signer Data BusinessName ContractorLicenseNumber ELECTRIC NIXON INC ELECTNI020CF GBMR ELECTRIC LLC GBMREEL815BJ Handyman Electric LLC HANDYEL911RU ABSOLUTE SECURITY ALARMS LLC ABSOLSA875C2 ABSOLUTE SECURITY ALARMS LLC ABSOLSA875C2 ABSOLUTE SECURITY ALARMS LLC ABSOLSA875C2 AJ HEATING & A/C LLC AJHEAHC804CQ ABSOLUTE SECURITY ALARMS LLC ABSOLSA875C2 Advanced Technlgy Htg/Clg LLC ADVANTH804CM BARKING DOG INC BARKIDI805CM NEURILINK LLC NEURIL*834MM NEURILINK LLC NEURIL*834MM NEURILINK LLC NEURIL*834MM NEURILINK LLC NEURIL*834MM EI&C SERVICES, LLC EICSESL807CA Limited Energy Contractor LLC LIMITEC807CM STURGEON ELECTRIC CO INC STURGEC853BL NEUMAN ELECTRIC INC NEUMAEI799K4 24 HOUR ELECTRIC INC 24HOUHE926NE SECURITY 101 SEATTLE SECUR1S812Q7 Page 1 of 956 09/23/2021 L&I Contractor Authorized Signer Data ContractorLicenseTypeCode ContractorLicenseTypeCodeDesc EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR EC ELECTRICAL CONTRACTOR Page 2 of 956 09/23/2021 L&I Contractor Authorized Signer Data BusinessTypeCode BusinessTypeCodeDesc UBI C Corporation 601846722 L Limited Liability Company 604380100 L Limited -
Lightweight Passenger Cars (Includes *Some* Amtrak, RDC, and Commuter Cars at This Time)
Lightweight Passenger Cars (includes *some* Amtrak, RDC, and commuter cars at this time) Road Car Name Type Manufacturer Model Number Builder Plan Lot Begin Dt This is a VERY dynamic NOTHING is set in If you have comments, .Note0 Document. It is constantly being concrete. please voice them! updated, and improved. This list contains lightweight car General Athearn, Tyco, Model Cars too Short Excluded information - and an effort has been Manufacturer Power, Tenshodo, Varney , made to include all manufacturers of Notes: Lifelike, Con-Cor shorties accurate represntations of the .Note1 prototype. Note: It is understood that not all models or Blue Line, JS Silversides, Cars long out of Excluded prototypes are included and none American Beauty, production, difficult to have been excluded for reasons Wrightline find .Note2 other than lack of information (or as indicated here). If you have information to add to or Generic, no prototype OK Streamliners, correct this effort - please feel free to Herkimer .Note3 contact me at no17 @ comcast . net (removing blanks). Note that cars purchased and Walthers Post-1998 NOTE: While the body converted may have different window production models: style of some …..the coinfigurations making the models Walthers cars may be underbody associated with the original owner accurate for a given details may .Note4 suspect for subsequent owners. prototype…… represent (This is especially true for Amtrak & generic AutoTrain) prototypes 3/27/2006 1:44 PM 1 of 339 Compiled By Members of the PCL List Lightweight Passenger Cars (includes *some* Amtrak, RDC, and commuter cars at this time) Road Car Name Type Manufacturer Model Number Builder Plan Lot Begin Dt As manufacturers change names, In some cases, the list becomes harder to keep when they current. -
2021 AIBA Catalogue of Results
2021 CATALOGUE OF RESULTS The Royal Agricultural Society of Victoria (RASV) thanks the following partners and supporters for their involvement. PRESENTING PARTNERS MAJOR SPONSORS TROPHY SPONSORS 2021 Catalogue of Results 2021 Catalogue of Results The Royal Agricultural Society of Victoria Limited The Royal Agricultural Society of Victoria Limited ABN 66 006 728 785 ACN 006 728 785 ABN 66 006 728 785 ACN 006 728 785 Melbourne Showgrounds Melbourne Showgrounds Epsom Road Epsom Road Ascot Vale VIC 3032 Ascot Vale VIC 3032 www.rasv.com.au www.rasv.com.au List of Office Bearers As at 01/05/2021 List of Office Bearers As at 01/05/2021 Patron Her Excellency the Hon Linda Dessau AC — Patron Her Excellency the Hon Linda Dessau AC — Governor of Victoria Governor of Victoria Board of Directors MJ (Matthew) Coleman (President) Board of Directors MJ (Matthew) Coleman (President) Dr. CGV (Catherine) Ainsworth (Deputy President) Dr. CGV (Catherine) Ainsworth (Deputy President) D (Darrin) Grimsey D (Darrin) Grimsey NE (Noelene) King OAM NE (Noelene) King OAM PJB (Jason) Ronald OAM PJB (Jason) Ronald OAM Dr. P (Peter) Hertan Dr. P (Peter) Hertan R (Robert) Millar R (Robert) Millar K (Kate) O'Sullivan K (Kate) O'Sullivan K (Kate) Fraser K (Kate) Fraser T (Tina) Savona T (Tina) Savona Chief Executive Officer B. Jenkins Chief Executive Officer B. Jenkins Company Secretary D. Ferris Company Secretary D. Ferris Advisory Group Members Craig Bowen Craig Bowen Advisory Group Members Justin Fox Justin Fox Warren Pawsey Warren Pawsey Tina Panoutsos Tina Panoutsos Billy Ryan Billy Ryan Competition Co-ordinator Damian Nieuwesteeg Competition Co-ordinator Damian Nieuwesteeg Email: [email protected] Email: [email protected] Australian International 3 Beer Awards Crafting award-winning beers for 30 years. -

Planning Department
PLANNING DEPARTMENT 970.668.4200 0037 Peak One Dr. | PO Box 5660 www.SummitCountyCO.gov Frisco, CO 80443 TEN MILE PLANNING COMMISSION AGENDA November 9, 2017 - 5:30 p.m. Buffalo Mountain Room, County Commons 0037 Peak One Dr., SCR 1005, Frisco, CO Commission Dinner: 5:00pm I. CALL TO ORDER II. ROLL CALL III. APPROVAL OF SUMMARY OF MOTIONS: September 14, 2017 IV. APPROVAL OF AGENDA: Additions, Deletions, Change of Order V. CONSENT AGENDA: None VI. PUBLIC HEARINGS: None VII. WORK SESSION ITEMS: PLN17-089 Copper Mountain Resort Major PUD Amendment (Housing) Work session for a Major PUD Amendment to the Copper Mountain Resort PUD to add Deed- Restricted Housing as an allowed use to Parcel 30 to facilitate up to 80 units of housing in the northern portion of the Alpine Parking Lot (an approximately 2.5 acre site), as well as add outdoor vendors as an allowed use to Parcels 6, 12, and 24. Additional sections of the PUD to be amended include parking, open space, and other amendments to accomplish the foregoing; Township 6 South, Range 79 West, Sections 25 and 26; Range 78 West, Sections 29, 30, and 32 VII. DISCUSSION ITEMS: Suggested Revisions for Consideration in Next Master Plan Update Countywide Planning Commission issues Follow-up of previous BOCC meeting Planning Commission Issues VIII. ADJOURNMENT * Allowance for Certain Site Plans to Be Placed on the Consent Agenda: Site plan reviews consisting of three (3) to a maximum of 12 multi-family units for the total development parcel or project may be placed on a Planning Commission’s “consent agenda”, which allows for expeditious review and approval of these smaller projects. -

0 1.5 3 4.5 6 0.75 Miles
k Brittle Silver Mountain Chief Mountain ek Recpa a don Vail w Cre th e an Lichen ado L Me C h Me r P ado w c e Co r d sh th ve o l t pa d n Lu oin c L s u u p e e e o ake R u F ! s M L or g ( b es H s o G o t Tiptop Peak t G e id M l le e s e n n aw o v a a n e d y r e E a a C T e 0 d r in Morgan Peak d o m r 7 i d d Y a I S w t o n a r 1 r e n l 8 C l r i w u o 0 a r i r P u r 4 o e m R o a awn e a m w 1 w e r H 0 W flo k r e 3 M d h m 9 T t e T t in a s r p t t r n M W c i n o n i t S e l s e n t n n e e M Silver Mountain R i i i e v s L w r F u o y o i R o a d P l l e o Independence Mountain r t S t g t D l Y P a Larson o K n e o a C l n o e h l U o n M k d c c S l u S n G a t n u C ll p n a o u o l s s n w e d rd a n l h U c n e e y o Aspen G a h i M l o i t i r l l e h t i o n W h a n a n ft f r C i e F T w a F e s e Alpine S h 7 P G g E 6 y w n g mil t o a i y d x t h R Creekside h S d o H o l re na a 6 ari J t a M h o e M alen id G 4 t G s C s 7 P m h a a t ile e tre h t r K M r S h e Ten d tion e 1 rea o 6 c e y Main i 2 Re h s s 3 5 s B t k F n D c t h il u th o t r t lon R o a h es n r cp d d ervo T n n i k Re r Recp a e 8 ath r e ail e N Ten Mile Tr J Bayview a G Bear Mountain t R M u u n 7 h i P o l l a i i n risc e c n F n t Lookup h rt i h n e i itk i M p P n s o s G e t e M a n P ford il s r el e ra o n i l B S n T Swan Mountain y i n o i n te s i a ia lla O ng o e e l i l Collier Mountain V id r k pr M Royal Mountain r d e J a S K h a u t M Bills Ranch y e on l a P Ir a l p B c l e e Unk e R (! nown B ile k T U Tra em -
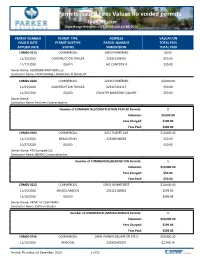
Permits Issued Fees Values No Voided Permits Town of Parker Date Range Between 11/1/2020 and 11/30/2020
Permits Issued Fees Values no voided permits Town of Parker Date Range Between 11/1/2020 and 11/30/2020 PERMIT NUMBER PERMIT TYPE ADDRESS VALUATION ISSUED DATE PERMIT SUBTYPE PARCEL NUMBER TOTAL FEES APPLIED DATE STATUS SUBDIVISION TOTAL PAID COM20-0215 COMMERCIAL 10653 PARKER RD $0.00 11/19/2020 CONSTRUCTION TRAILER 223315303010 $50.00 11/17/2020 ISSUED MC CLINTOCK II $50.00 Owner Name: HEIRBORN PARTNERS LLC Contractor Name: FRONTRANGE LANDSCAPE & NURSERY COM20-0220 COMMERCIAL 12245 PARKER RD $4,000.00 11/23/2020 CONSTRUCTION TRAILER 223327401117 $50.00 11/20/2020 ISSUED COUNTRY MEADOWS SQUARE $50.00 Owner Name: Contractor Name: Facilities Contracting Inc Number of COMMERCIAL/CONSTRUCTION TRAILER Permits: 2 Valuation: $4,000.00 Fees Charged: $100.00 Fees Paid: $100.00 COM20-0200 COMMERCIAL 3471 FILBERT AVE $10,000.00 11/12/2020 DEMOLITION 223306300003 $50.00 10/27/2020 ISSUED $50.00 Owner Name: 470 Compark LLC Contractor Name: BEMAS Construction Inc Number of COMMERCIAL/DEMOLITION Permits: 1 Valuation: $10,000.00 Fees Charged: $50.00 Fees Paid: $50.00 COM20-0212 COMMERCIAL 19552 MAINSTREET $10,000.00 11/19/2020 MISCELLANEOUS 223322100032 $599.06 11/16/2020 ISSUED $599.06 Owner Name: ABDALLA I SULEIMAN Contractor Name: Collin m Stauter Number of COMMERCIAL/MISCELLANEOUS Permits: 1 Valuation: $10,000.00 Fees Charged: $599.06 Fees Paid: $599.06 COM20-0196 COMMERCIAL 19641 PARKER SQUARE DR STE G $59,000.00 11/10/2020 REMODEL 223322402007 $2,346.14 Printed: Thursday, 03 December, 2020 1 of 51 Permits Issued Fees Values no voided permits Town -

Ranger District Leadville National Forest San Isabel
e r u t l u c i r g A f o t n e m t r a p e D s e t a t S d e t i n U 370000 380000 390000 400000 R82W 106°30'0"W R81W 106°22'30"W R80W 106°15'0"W R79W 106°7'30"W e c i v r e S t s e r o F PURPOSE AND CONTENTS OPERATOR R78W 19 21 9 1 0 2 , . T C O O D A R O L O C OF THIS MAP 24 20 22 Crystal Peak 24 ) RESPONSIBILITIES 23 T he designations shown on this motor vehicle use 23 Mount Powell Father Dyer Peak) 24 19 20 ) 19 20 21 22 O perating a motor vehicle on National Forest 21 20 map (MV U M) were made by the responsible official 22 19 23 19 21 24 20 24 pursuant to 36 CFR 212.51; are effective as of the date System roads, National Forest System trails, and in 21 22 23 Pacific Peak areas on National Forest System lands carries a ) on the front cover of this MV U M; and will remain in effect until superceded by the nex t year's MV U M. greater responsibility than operating that vehicle in a S U city or other developed setting. Not only must you M 27 s r o o d t u O t a e r G s ' a c i r e m A 26 25 30 28 30 25 29 Atlantic Peak know and follow all applicable traffic laws, you need to 29 29 28 M 26 30 26 25 30 E ) 28 28 27 27 26 25 A IT show concern for the environment as well as other 29 e r u t c i P G C forest users.