Influence of Bedrock Geology and Tree Species Composition on Stream Nitrate Concentrations in Mid-Appalachian Forested Watersheds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Integrating the MAPS Program Into Coordinated Bird Monitoring in the Northeast (U.S
Integrating the MAPS Program into Coordinated Bird Monitoring in the Northeast (U.S. Fish and Wildlife Service Region 5) A Report Submitted to the Northeast Coordinated Bird Monitoring Partnership and the American Bird Conservancy P.O. Box 249, 4249 Loudoun Avenue, The Plains, Virginia 20198 David F. DeSante, James F. Saracco, Peter Pyle, Danielle R. Kaschube, and Mary K. Chambers The Institute for Bird Populations P.O. Box 1346 Point Reyes Station, CA 94956-1346 Voice: 415-663-2050 Fax: 415-663-9482 www.birdpop.org [email protected] March 31, 2008 i TABLE OF CONTENTS EXECUTIVE SUMMARY .................................................................................................................... 1 INTRODUCTION .................................................................................................................................. 3 METHODS ............................................................................................................................................. 5 Collection of MAPS data.................................................................................................................... 5 Considered Species............................................................................................................................. 6 Reproductive Indices, Population Trends, and Adult Apparent Survival .......................................... 6 MAPS Target Species......................................................................................................................... 7 Priority -
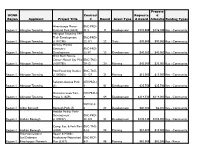
Keystone Fund Projects by Applicant (1994-2017) Propose DCNR Contract Requeste D Region Applicant Project Title # Round Grant Type D Award Allocatio Funding Types
Keystone Fund Projects by Applicant (1994-2017) Propose DCNR Contract Requeste d Region Applicant Project Title # Round Grant Type d Award Allocatio Funding Types Alverthorpe Manor BRC-PRD- Region 1 Abington Township Cultural Park (6422) 11-3 11 Development $223,000 $136,900 Key - Community Abington Township TAP Trail- Development BRC-PRD- Region 1 Abington Township (1101296) 22-171 22 Trails $90,000 $90,000 Key - Community Ardsley Wildlife Sanctuary- BRC-PRD- Region 1 Abington Township Development 22-37 22 Development $40,000 $40,000 Key - Community Briar Bush Nature Center Master Site Plan BRC-TAG- Region 1 Abington Township (1007785) 20-12 20 Planning $42,000 $37,000 Key - Community Pool Feasibility Studies BRC-TAG- Region 1 Abington Township (1100063) 21-127 21 Planning $15,000 $15,000 Key - Community Rubicam Avenue Park KEY-PRD-1- Region 1 Abington Township (1) 1 01 Development $25,750 $25,700 Key - Community Demonstration Trail - KEY-PRD-4- Region 1 Abington Township Phase I (1659) 4 04 Development $114,330 $114,000 Key - Community KEY-SC-3- Region 1 Aldan Borough Borough Park (5) 6 03 Development $20,000 $2,000 Key - Community Ambler Pocket Park- Development BRC-PRD- Region 1 Ambler Borough (1102237) 23-176 23 Development $102,340 $102,000 Key - Community Comp. Rec. & Park Plan BRC-TAG- Region 1 Ambler Borough (4438) 8-16 08 Planning $10,400 $10,000 Key - Community American Littoral Upper & Middle Soc/Delaware Neshaminy Watershed BRC-RCP- Region 1 Riverkeeper Network Plan (3337) 6-9 06 Planning $62,500 $62,500 Key - Rivers Keystone Fund Projects by Applicant (1994-2017) Propose DCNR Contract Requeste d Region Applicant Project Title # Round Grant Type d Award Allocatio Funding Types Valley View Park - Development BRC-PRD- Region 1 Aston Township (1100582) 21-114 21 Development $184,000 $164,000 Key - Community Comp. -

PRIMITIVE CAMPING in Pennsylvania State Parks and Forests 11/2014
PRIMITIVE CAMPING in Pennsylvania State Parks and Forests 11/2014 What is Primitive Camping? Primitive camping is a simplistic style of camping. Campers hike, pedal or paddle to reach a location and spend the night without the presence of developed facilities. This primitive camping experience takes place off the beaten path, where piped water, restrooms and other amenities are not provided. You pack in all you need, exchanging a few conveniences for the solitude found in the back country setting. Fresh air, fewer people and out-of-the-way natural landscapes are some of the benefits of primitive camping. Once off the beaten path, however, additional advantages begin to surface such as a deeper awareness and greater appreciation of the outdoor world around you. Primitive camping also builds outdoor skills and fosters a gratifying sense of self-sufficiency. Where to Camp Pennsylvania has 2.2 million acres of state forest land with 2,500 miles of trails and 5,132 miles of Camping at rivers and streams winding through it. Hiking, biking and multi-use trails traverse most state forest districts and six districts have designated water trails that transect state forest land. Forest Districts State parks are not open to primitive camping. However, with the exception of William Penn State Forest, all state forest districts are open to this activity. Camping is not permitted in designated STATE FOREST DISTRICTS: natural areas or at vistas, trail heads, picnic areas and areas that are posted closed to camping. Bald Eagle State Forest (570) 922-3344 Contact a forest district office for specific information, maps and Camping Permits (if needed). -

FALL FOLIAGE REPORT October 1 – October 7, 2020
Pennsylvania Department of Conservation and Natural Resources Bureau of Forestry PENNSYLVANIA WEEKLY FALL FOLIAGE REPORT October 1 – October 7, 2020 TIOGA CAMERON BRADFORD To predict peak season from the colors, use these guidelines: ➢ Dark Green/No Change= peak is three weeks to a month away ➢ Light Green/Starting to Change= peak is approximately two to three weeks away ➢ Yellow/Approaching Best Color= peak is approximately one week away ➢ Orange/Best Color= foliage is peaking this week ➢ Red/Starting to Fade= still some nice color but won’t last more than a few more days ➢ Brown/Past Peak= all or nearly all color is gone Statewide Fall Foliage Overview What a difference a week makes! Cold and frosty nights and very dry conditions have ushered in marked changes across the northern tier, where foliage is at its spectacular peak. Sugar and red maples are the stars of the show, contrasting beautifully with the still-green oak canopies. Another cold snap could jumpstart much of the Appalachian and Allegheny mountain regions, setting the table for back to back weeks of fantastic fall foliage viewing opportunities across wide swaths of the commonwealth. Northwestern Region The district manager in Cornplanter State Forest District (Warren, Erie counties) reports that cooler nights have spurred fall colors in northwest Pennsylvania. Many oaks are still quite green, but maples (sugar and red) are displaying brilliant colors. Aspen, hickory, and birch are continuing to color the landscape with warm yellow hues. Route 6 to Chapman State Park is a recommended fall foliage corridor in Warren County. Exciting fall color can be found at Chapman State Park. -

HISTORY of PENNSYLVANIA's STATE PARKS 1984 to 2015
i HISTORY OF PENNSYLVANIA'S STATE PARKS 1984 to 2015 By William C. Forrey Commonwealth of Pennsylvania Department of Conservation and Natural Resources Office of Parks and Forestry Bureau of State Parks Harrisburg, Pennsylvania Copyright © 2017 – 1st edition ii iii Contents ACKNOWLEDGEMENTS ...................................................................................................................................... vi INTRODUCTION ................................................................................................................................................. vii CHAPTER I: The History of Pennsylvania Bureau of State Parks… 1980s ............................................................ 1 CHAPTER II: 1990s - State Parks 2000, 100th Anniversary, and Key 93 ............................................................. 13 CHAPTER III: 21st CENTURY - Growing Greener and State Park Improvements ............................................... 27 About the Author .............................................................................................................................................. 58 APPENDIX .......................................................................................................................................................... 60 TABLE 1: Pennsylvania State Parks Directors ................................................................................................ 61 TABLE 2: Department Leadership ................................................................................................................. -

PENNSYLVANIA NWTF Super Fund Spending Exceeds $5 Million!
PENNSYLVANIA NWTF Super Fund Spending Exceeds $5 Million! Pennsylvania’s 86 local NWTF chapters and more than 12,500 members have raised and spent $5,703,959 through the state’s Super Fund on habitat management, wild turkey management, turkey hunter safety education, land acquisitions and NWTF outreachManagement programs since 1985. Habitat enhancement projects have impacted more than Spent $23,960 on wild turkey management. Habitat Enhancement 257,895 acres within the state. • 16,811 acres of wildlife openings • operation of wild turkey check stations • 5 acres of prescribed burning • hunter success and satisfaction surveys Spent $3,156,743 on habitat • completion of wild turkey management plans improvement projects. Purchased equipment for • support of law enforcement activities • maintenance/development of brood habitat habitat management including: Purchased equipment to • wildlife openings • 1 trailer • prescribed fire • 10 grain drills support law enforcement and • tree planting • 3 tractors management activities including: • riparian restoration • 5 sprayers • ATV’s • water development projects • 3 cultipackers • weight scales for check stations • control of invasive plant species • 4 planters • survey/surveillance cameras • support for seed subsidy • 3 spreaders • gates to limit vehicular access and conservation seed programs • 4 disk/harrows • 2 mowers Land Purchase Projects were completed on: Spent $340,650 to purchase • Allegheny National Forest • Hills Creek State Park • Prince Gallitzin State Park 27,940 acres of land or • Archbald Pothole State Park • Jacobsburg State Park • Raystown Lake Management Area conservation easements to • Bald Eagle State Forest • Kettle Creek State Park • Rocky Ridge State Park preserve wildlife habitat and • Black Moshannon State Park • Lackawana State Forest • Ryerson Station State Park provide additional hunting areas. -

Pennsylvania Year-Round Seasonal Events 2016
PENNSYLVANIA YEAR-ROUND AND SEASONAL EVENTS 2016 KEYSTONE STATE VOLKSSPORT ASSOCIATION www.ksva.net 2134 Barley Drive Quakertown, PA 18951 www.ava.org GENERAL INFORMATION Awards for completing the IVV achievement books consist of a lapel pin, patch and certificate acknowledging completion of each level of accomplishment. VOLKSSPORTING is an adventure in health, recreation, fellowship and fun. Volkssporting literally translates as “sport Event and distance books are $6.00 each. Some clubs have of the people”. The program consists of organized events them for sale in their start box. Other clubs include insert designed to appeal to people of all ages. These are cards for your use. A New Walker Packet is available for noncompetitive events. This is exercise at your own pace. $5.00 that includes an event book, a distance book and three The volkssporting philosophy recognizes that everyone who coupons that are good for IVV credit. participates is a winner and eligible for an award as Procedures: At each event start, each participant must described in the event brochures. register to obtain a start card which must be filled out. This Most events are walks, but sports such as bicycling, card must be carried, marked at checkpoints and returned at swimming and cross-country skiing are also included. Each the completion of the trail. All participants must sign the event has a pre-marked trail on which to enjoy the company Waiver of Liability. of friends, family and fellow volkssporters. Consult the event publication for a description of the trail, rating, dates, and times. Participate in the event during IVV/AVA Sanction: All events are sanctioned by the daylight hours only. -

FALL FOLIAGE REPORT October 8 – October 14, 2020
Pennsylvania Department of Conservation and Natural Resources Bureau of Forestry PENNSYLVANIA WEEKLY FALL FOLIAGE REPORT October 8 – October 14, 2020 TIOGA CAMERON BRADFORD To predict peak season from the colors, use these guidelines: ➢ Dark Green/No Change= peak is three weeks to a month away ➢ Light Green/Starting to Change= peak is approximately two to three weeks away ➢ Yellow/Approaching Best Color= peak is approximately one week away ➢ Orange/Best Color= foliage is peaking this week ➢ Red/Starting to Fade= still some nice color but won’t last more than a few more days ➢ Brown/Past Peak= all or nearly all color is gone Statewide Fall Foliage Overview Gorgeous color continues to abound throughout Penn’s Woods! While some northern counties have begun to fade, central forests are now stealing the show. Awesome color can be found from northwestern Pennsylvania through the Alleghenies and central Appalachians, to the Pocono region. Forecasted seasonable temperatures should facilitate a gradual transition of color throughout southern areas of the commonwealth, affording fantastic viewing opportunities into late October. Northwestern Region The district manager in Cornplanter State Forest District (Warren, Erie counties) stated that cool nights have brought on a splendid array of colors on the hillsides of northwestern Pennsylvania. Although peak is still more than a week away, every shade of yellow, red, orange, and brown is represented in the forested landscape. It’s a great time to get outdoors and take advantage of the autumn experience and the many opportunities to walk forested trails carpeted in newly fallen leaves! To view and enjoy the fall foliage by vehicle, consider taking routes 666, 62, 59, or 321. -

Dcnr 20033722
Pennsylvania Department of Conservation and Natural Resources Bureau of Forestry PENNSYLVANIA WEEKLY FALL FOLIAGE REPORT October 4 – October 10, 2018 New Map Format You will notice the fall foliage map is different this year. We hope you find it more useful and attractive. A few words follow on how to interpret the new format. TIOGA CAMERON BRADFORD The new Fall Foliage Map is based on a color-code system, where brighter colors essentially mean more vibrant foliage. To predict peak season from the colors, use these guidelines: ➢ Dark Green/No change= peak is approximately one month away ➢ Light Green/Starting to Change= peak is approximately three weeks away ➢ Yellow/Approaching Best Color= peak is approximately one week away ➢ Orange/Best Color= foliage is peaking this week ➢ Red/Starting to Fade= still some nice color but won’t last more than a few more days ➢ Brown/Past Peak= all or nearly all color is gone Statewide Fall Foliage Overview Above-average temperatures last week slowed the foliage transition throughout the commonwealth. Foresters in northern and southwestern Pennsylvania are reporting localized defoliations of some maples, cherries, and oaks due to heavy wind, rain, and fungi. Most foliage in southern Pennsylvania is unchanged from last week, but most of the state’s forests are showing signs of the season. Northeastern counties continue to boast the best color in the state, however substantial foliage progress has been noted from Mercer eastward through Clearfield counties. Long-range weather forecasts call for a considerable cooldown in roughly one week, which should accelerate the foliar transition throughout the majority of Pennsylvania. -

Appendix E Conservation Lands Crossed by Nisource
APPENDIX E CONSERVATION LANDS CROSSED BY NISOURCE FACILITIES Appendix E – Conservation Lands Crossed by NiSource Facilities State Property Name Owner Type Delaware Bechtel Park Local Delaware Knollwood Park Local Delaware Naamans Park East Local Delaware Naamans Park North Local Indiana Eagle Lake Wetlands Conservation Area State Indiana Kingsbury Fish and Wildlife Area State Indiana Mallard Roost Wetland Conservation Area State Indiana St. John Prairie State Indiana Deep River County Park Local Indiana Northside Park Local Indiana Oak Ridge Prairie County Park Local Indiana Gaylord Butterfly Area NGO Kentucky Carr Creek State Park Federal Kentucky Daniel Boone National Forest Federal Kentucky Dewey Lake Wildlife Management Area Federal Kentucky Green River Lake Wildlife Management Area Federal Kentucky Jenny Wiley State Resort Park Federal Kentucky Lexington-Blue Grass Army Depot Federal Kentucky Carr Fork Lake Wildlife Management Area State Kentucky Central Kentucky Wildlife Management Area State Kentucky Dennis-Gray Wildlife Management Area State Kentucky Floracliff State Nature Preserve State Louisiana Bayou Teche National Wildlife Refuge Federal Louisiana Cameron Prairie National Wildlife Refuge Federal Louisiana Grand Cote National Wildlife Refuge Federal Louisiana Lacassine National Wildlife Refuge Federal Louisiana Mandalay National Wildlife Refuge Federal Louisiana Sabine National Wildlife Refuge Federal Louisiana Tensas River National Wildlife Refuge Federal Louisiana Big Lake Wildlife Management Area State Louisiana Boeuf Wildlife -

Cowans Gap State Park History
A Pennsylvania Recreational Guide for State Park Cowans Gap Cowans Gap Pennsylvania State Parks Mission The primary purpose of Pennsylvania state parks is to provide opportunities for enjoying healthful outdoor recreation and serve as outdoor classrooms for environmental education. In meeting these purposes, the conservation of the natural, scenic, aesthetic, and historical values of parks should be given first consideration. Stewardship responsibilities should be carried out in a way that protects the natural outdoor experience for the enjoyment of current and future generations. visitPAparks Printed on recycled paper 2017 COWANS GAP STATE PARK HISTORY Cowans Gap State Park is a 1,085-acre park Directions FROM THE SOUTH: US 30 to PA 75 north The history of Cowans Gap State Park is one from opposite political parties, the couple Kalbach and his partner Charles Spangler, of in the beautiful Allens Valley of Fulton at Fort Loudon to Richmond Furnace and of war, forbidden love, and hard work. eloped several years later and headed for McConnellsburg, operated a portable sawmill County. A 42-acre lake, two campgrounds, The park is north of US 30 between follow signs to the park. Kentucky. The family story relates that while in the valley, cutting mainly pine and oak. The Famous Roads rustic Civilian Conservation Corps (CCC) Chambersburg and McConnellsburg. crossing the Conococheague Creek near final cut was completed in 1907, leaving a Reservations The French and Indian War began in 1754 built cabins, and many hiking trails are prime Fort Loudon, their wagon broke down. John landscape of over-cut forests and erosion. FROM THE WEST: I-76 to Exit 180, Fort as the French and British fought for the attractions. -

Visit PATC's New Jewels in Pennsylvania Product Review
ISSN 098—8154 The Newsletter of the Potomac Appalachian Trail Club Volume 34, Number 4 118 Park Street, S.E., Vienna, VA 22180-4609 April 2005 http://www.patc.net Visit PATC’s New Jewels in Pennsylvania unday, April 10 is your chance to see impres- Ssive additions to PATC’s portfolio along the Tuscarora Trail in South-central Pennsylvania. 54 Acre Tract of Land Purchased PATC has completed the acquisition of 54 acres of land north of Cowans Gap in Central Pennsylvania. The almost completely wooded tract shares a long boundary with the Buchanan State Forest; the Tuscarora Trail runs along the top of this tract, and a major trout stream, Aughwick Creek, crosses the land. A log cabin, to be known as the Silberman Cabin, will be constructed here beginning in late spring or early summer. PATC members and friends are invited to see and explore this new property at an ‘Open House’ on Sunday, April 10, from 10:00 a.m. until 2:00 p.m. To reach the property, take U.S. 30 to the top of Tuscarora Mountain, between Fort Louden and McConnellsburg, Smith Photo by Walt Group out in the snow scouting for the future Silberman Cabin site on the newly See Jewels page purchased central Pennsylvania tract Pictured in foreground is Charlie Graf Product Review: TrailMaster ‘Shenandoah 3500’ In This Issue . Council Fire . .2 long, cold winter spent indoors can mean setup, although less clear for operation. The Tom’s Trail Talk . .3 Aan arduous task of getting back in shape unit requires normal 110V AC power and Volunteers Appointed .