Non-Invasive Spectroscopic Investigations of Maya Blue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Great Tekhelet Debate—Blue Or Purple? Baruch and Judy Taubes Sterman
archaeological VIEWS The Great Tekhelet Debate—Blue or Purple? Baruch and Judy Taubes Sterman FOR ANCIENT ISRAELITES, TEKHELET WAS writings of rabbinic scholars and Greek and Roman God’s chosen color. It was the color of the sumptu- naturalists had convinced Herzog that tekhelet was a ous drapes adorning Solomon’s Temple (2 Chroni- bright sky-blue obtained from the natural secretions cles 3:14) as well as the robes worn by Israel’s high of a certain sea snail, the Murex trunculus, known to priests (Exodus 28:31). Even ordinary Israelites produce a dark purple dye.* were commanded to tie one string of tekhelet to But the esteemed chemist challenged Herzog’s the corner fringes (Hebrew, tzitzit) of their gar- contention: “I consider it impossible to produce a ments as a constant reminder of their special rela- pure blue from the purple snails that are known to Tekhelet was tionship with God (Numbers 15:38–39). me,” Friedländer said emphatically.1 But how do we know what color the Biblical writ- Unfortunately, neither Herzog nor Friedländer God’s chosen ers had in mind? While tekhelet-colored fabrics and lived to see a 1985 experiment by Otto Elsner, a color. It colored clothes were widely worn and traded throughout the chemist with the Shenkar College of Fibers in Israel, ancient Mediterranean world, by the Roman period, proving that sky-blue could, in fact, be produced the drapes donning tekhelet and similar colors was the exclusive from murex dye. During a specific stage in the dyeing of Solomon’s privilege of the emperor. -

Western Blotting Guidebook
Western Blotting Guidebook Substrate Substrate Secondary Secondary Antibody Antibody Primary Primary Antibody Antibody Protein A Protein B 1 About Azure Biosystems At Azure Biosystems, we develop easy-to-use, high-performance imaging systems and high-quality reagents for life science research. By bringing a fresh approach to instrument design, technology, and user interface, we move past incremental improvements and go straight to innovations that substantially advance what a scientist can do. And in focusing on getting the highest quality data from these instruments—low backgrounds, sensitive detection, robust quantitation—we’ve created a line of reagents that consistently delivers reproducible results and streamlines workflows. Providing scientists around the globe with high-caliber products for life science research, Azure Biosystems’ innovations open the door to boundless scientific insights. Learn more at azurebiosystems.com. cSeries Imagers Sapphire Ao Absorbance Reagents & Biomolecular Imager Microplate Reader Blotting Accessories Corporate Headquarters 6747 Sierra Court Phone: (925) 307-7127 Please send purchase orders to: Suite A-B (9am–4pm Pacific time) [email protected] Dublin, CA 94568 To dial from outside of the US: For product inquiries, please email USA +1 925 307 7127 [email protected] FAX: (925) 905-1816 www.azurebiosystems.com • [email protected] Copyright © 2018 Azure Biosystems. All rights reserved. The Azure Biosystems logo, Azure Biosystems™, cSeries™, Sapphire™ and Radiance™ are trademarks of Azure Biosystems, Inc. More information about Azure Biosystems intellectual property assets, including patents, trademarks and copyrights, is available at www.azurebiosystems.com or by contacting us by phone or email. All other trademarks are property of their respective owners. -

Shades of Blue
Episode # 101 Script # 101 SHADES OF BLUE “Pilot” Written by Adi Hasak Directed by Barry Levinson First Network Draft January 20th, 2015 © 20____ Universal Television LLC ALL RIGHTS RESERVED. NOT TO BE DUPLICATED WITHOUT PERMISSION. This material is the property of Universal Television LLC and is intended solely for use by its personnel. The sale, copying, reproduction or exploitation of this material, in any form is prohibited. Distribution or disclosure of this material to unauthorized persons is also prohibited. PRG-17UT 1 of 1 1-14-15 TEASER FADE IN: INT. MORTUARY PREP ROOM - DAY CLOSE ON the Latino face of RAUL (44), both mortician and local gang leader, as he speaks to someone offscreen: RAUL Our choices define us. It's that simple. A hint of a tattoo pokes out from Raul's collar. His latex- gloved hand holding a needle cycles through frame. RAUL Her parents chose to name her Lucia, the light. At seven, Lucia used to climb out on her fire escape to look at the stars. By ten, Lucia could name every constellation in the Northern Hemisphere. (then) Yesterday, Lucia chose to shoot heroin. And here she lies today. Reveal that Raul is suturing the mouth of a dead YOUNG WOMAN lying supine on a funeral home prep table. As he works - RAUL Not surprising to find such a senseless loss at my doorstep. What is surprising is that Lucia picked up the hot dose from a freelancer in an area I vacated so you could protect parks and schools from the drug trade. I trusted your assurance that no one else would push into that territory. -

Prolific Pigmentfinal4.19.20 Copy
Proliic pigment Create your own unique color! Summary: Throughout history, people have used pigment to express them- selves. How do you make a pigment and what could you use it for? What kind of paint can you create using pigments found at home? Guiding Questions: What are pigments? Where do pigments come from? What could you use pigments for? Experience Goals: • Explore how pigments are made and used. • Make your own pigment and turn it into paint. Supplies: • Pigment Info Sheet • Fresh or Freeze-dried Blueberries (pigment material) • Water (binding material) • Coloring sheet (page 5) • Paintbrush and paint cup • Mortar and Pestle (or another grinding tool, like a bowl and large spoon) • A space you can get messy in! 1. Steps: 1. Explore Pigments a. Explore the Pigment Info Sheet to learn about pigment and how it is used. b. Think about what could create pigment in your home. Is there anything in your kitchen? How about colorful plants outside? c. We will use blueberries to make our color! You can ind the recipe in Step 3. What other colors could you create? What would you paint with them? 2. Make Your Pigment a. Gather your pigment material. Usually a pigment used in painting will be powdered, but can also be in juice form. Crushed up freeze dried fruits like blueberries make for an excellent pigment powder! b. Grind or mash up your pigment material. If using fresh blueberries, mash them then strain out the juice using a kitchen strainer. With frozen or freeze dried blueberries, use a mortar and pestle (or similar items like a bowl and large spoon) to grind them into a ine powder. -

Three Shades of Blue: Air Force Culture and Leadership
Three Shades of Blue: Air Force Culture and Leadership Bernadette Pothan Submitted in fulfilment of the requirement of the degree of Master of Philosophy University College, the University of New South Wales 2013 Three Shades of Blue: Air Force Culture and Leadership ABSTRACT The study explores unconstructive ideas of power in the military. In the thesis doctrine is seen to promote ideas of power over others under cover of the language of leadership. The study explains how Australian Defence Doctrine Publication 00.6: Leadership in the Australian Defence Force confuses ideas of command and leadership, and asserts that in doctrine unconstructive ideas of power have engulfed ideas of leadership. More than published text, doctrine is understood to describe ideas which have pervasive cultural meaning and impact. The thesis explores how acculturated myths of power are causally relevant to air accidents, decayed maintenance standards, and the prejudice borne by women in the Service. The so- called ‘warrior culture’ is interrogated as the rationalisation of unconstructive power and the aggregated risk which follows on its heels. The focus of the study is narrowed to the Royal New Zealand Air Force, and explicit attention is devoted to the ‘characters’ which give life to unhelpful elements of the ‘warrior culture’. The thesis unpacks the ‘characters’ (‘Aircrew’, ‘Maintenance Crew’, and ‘Support Crew’) and explores how unconstructive ideas of power are discernable in Air Force culture. ‘Aircrew’ are seen to infuse and dominate every aspect of Air Force life. ‘Maintenance Crew’ is a symbol for the Air Force maintenance culture, revealing the controlling influence and the practical repercussions of the ‘Aircrew’ myth. -

Color Matters
Color Matters Color plays a vitally important role in the world in which we live. Color can sway thinking, change actions, and cause reactions. It can irritate or soothe your eyes, raise your blood pressure or suppress your appetite. When used in the right ways, color can even save on energy consumption. As a powerful form of communication, color is irreplaceable. Red means "stop" and green means "go." Traffic lights send this universal message. Likewise, the colors used for a product, web site, business card, or logo cause powerful reactions. Color Matters! Basic Color Theory Color theory encompasses a multitude of definitions, concepts and design applications. There are enough to fill several encyclopedias. However, there are basic categories of color theory. They are the color wheel and the color harmony. Color theories create a logical structure for color. For example, if we have an assortment of fruits and vegetables, we can organize them by color and place them on a circle that shows the colors in relation to each other. The Color Wheel A color wheel is traditional in the field of art. Sir Isaac Newton developed the first color wheel in 1666. Since then, scientists and artists have studied a number of variations of this concept. Different opinions of one format of color wheel over another sparks debate. In reality, any color wheel which is logically arranged has merit. 1 The definitions of colors are based on the color wheel. There are primary colors, secondary colors, and tertiary colors. Primary Colors: Red, yellow and blue o In traditional color theory, primary colors are the 3 colors that cannot be mixed or formed by any combination of other colors. -

Indigo Blue Cas N°: 482-89-3
OECD SIDS INDIGO BLUE FOREWORD INTRODUCTION INDIGO BLUE 3H-Indol-3-one, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro CAS N°: 482-89-3 UNEP PUBLICATIONS 1 OECD SIDS INDIGO BLUE SIDS Initial Assessment Report For SIAM 2 Paris, France, 4-6 July 1994 1. Chemical Name: 3H-Indol-3-one, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2- dihydro- (Indigo Blue) 2. CAS Number: 482-89-3 3. Sponsor Country: Japan National SIDS Contact Point in Sponsor Country: Mr. Yasuhisa Kawamura, Ministry of Foreign Affairs, Japan 4. Shared Partnership with: 5. Roles/Responsibilities of the Partners: • Name of industry sponsor /consortium • Process used 6. Sponsorship History • How was the chemical or As a high priority chemical for initial assessment, Indigo Blue category brought into the was selected in the framework of OECD HPV Chemicals OECD HPV Chemicals Programme. Programme ? SIDS Dossier and Testing Plan were reviewed at a SIDS Review Meeting in 1993. At SIAM-2, the conclusion was approved with comments. Comments at SIAM-2: Rearrangement of the documents. 7. Review Process Prior to the SIAM: 8. Quality check process: 9. Date of Submission: Date of Circulation: March 1994 10. Date of last Update: 11. Comments: 2 UNEP PUBLICATIONS OECD SIDS INDIGO BLUE SIDS INITIAL ASSESSMENT PROFILE CAS No. 482-89-3 3H-Indol-3-one, 2-(1,3-dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro- Chemical Name (Indigo Blue) O H N Structural Formula N O H CONCLUSIONS AND RECOMMENDATIONS It is currently considered of low potential risk and low priority for further work. -

Title: Indigo in the Modern Arab World Fulbright Hays Oman and Zanzibar
Title: Indigo in the Modern Arab World Fulbright Hays Oman and Zanzibar Program June-July 2016 Author: Victoria Vicente School: West Las Vegas Middle School, New Mexico Grade Level: 7-10 World History, United States History Purpose: This lesson is an overview of the geography of Oman and to find out more about the Burqa in Oman. There are examples provided, artwork examples, a photo analysis, an indigo dyeing activity, and readings from, “Language of Dress in the Middle East.” Activate knowledge of the region by asking students questions, for example: What is the color of the ocean? The sky? What does the color blue represent? Have you ever heard the word Burqa? Can you find the Indian Ocean on a Map? Oman? Are you wearing blue jeans? Time: 150 minutes for the lesson Extension Activity: Three days for the indigo dyeing process. Required Materials: Map of the Indian Ocean Region, Map of Oman, including Nizwa, Bahla, and Ibri, Internet, Elmo, Projector, Timeline of Indigo in Oman, show the video clips about dyeing indigo, have the students fill out the photo analysis sheets, and then for the extension activity, spend at least three days or 15 minutes per class for a week, dyeing the material. Resources: 1. Map of Oman and the Indian Ocean region http://geoalliance.asu.edu/maps/world http://cmes.arizona.edu 2. Video clips for background information: Http://www.youtube.com/watch?v=pG1zd3b7q34 http://www.youtube.com/watch?v=7z6B7ismg3k http://www.youtube.com/watch?v=Ay9g6ymhysA&feature=related 3. National Archives Photo Analysis Form or create your own. -
Placement Analysis - 2019 Batch
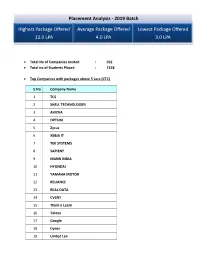
Placement Analysis - 2019 Batch Highest Package Offered Average Package Offered Lowest Package Offered 12.0 LPA 4.0 LPA 3.0 LPA Total No of Companies Invited : 502 Total no of Students Placed : 1328 Top Companies with packages above 5 Lacs (CTC) S.No Company Name 1 TCS 2 SHELL TECHNOLOGIES 3 AVIZVA 4 OPTUM 5 Zycus 6 XEBIA IT 7 TEK SYSTEMS 8 SAPIENT 9 MANN INDIA 10 HYUNDAI 11 YAMAHA MOTOR 12 RELIANCE 13 REAL DATA 14 CVENT 15 Think n Learn 16 Tolexo 17 Google 18 Dyson 19 United Lex 20 WIPRO WASE 21 HSBC 22 Accolite 23 Novanet 24 Foresight 25 Blue Star 26 RMS 27 Samsung 28 Amazon 29 Keyence 30 Veris Top Payers /Dream Companies Company Name CTC (LPA) INDIAN AIR FORCE 12 GOOGLE 12 HSBC 12 SIRION LABS 12 NATIONAL INSTRUMENTS 12 SHELL TECHNOLOGIES 10 THINK N LEARN 10 ACCOLITE 10 VMWARE 9.12 TCS (CODEVITA) 7.5 HYUNDAI 7.5 NOVANET 7 ZYCUS 6.5 XEBIA IT 6 MANN INDIA TECHNOLOGIES 6 RELIANCE 6 REAL DATA 6 FORESIGHT 6 BLUE STAR 6 SAMSUNG 6 AMAZON 6 KEYENCE 6 WIPRO WASE 5.57 OPTUM 5.4 SAPIENT 5.3 AVIZVA 5.25 DYSON 5.07 YAMAHA 5 CVENT 5 TOLEXO 5 UNITED LEX 5 PFIZER 5 LAMBORGINI 5 Top Recruiters Company Name Final Placed ACCENTURE 309 TCS 120 WIPRO TECHNOLOGIES 70 THINK N LEARN 40 ICONMA 39 NEC TECHNOLOGIES 29 AMAZON 52 FLIPKART 25 RELIANCE 23 MANN INDIA TECHNOLOGIES 21 SOPRA STERIA 20 HEXAWARE 16 HCL TECHNOLOGIES 23 SAMSUNG 28 ERICSSON 15 NTT DATA 33 Top IT Companies: S.No Company Name 1 NEC 2 TATA TECHNOLOGIES 3 WIPRO 4 ACCENTURE 5 HEXAWARE 6 OPTUM 7 NIIT 8 GRAPECITY 9 TCS 10 MPHASIS 11 SAPIENT 12 NEWGEN 13 ACCENTURE 14 Gemalto 15 Google 16 Genpact -

Japan Blues: Ao, Ai, Midori, Aizome, Ao-Ja-Shin. in Harkness, R
CLARKE, J. 2017. Japan blues: ao, ai, midori, aizome, ao-ja-shin. In Harkness, R. (ed.) An unfinished compendium of materials. Aberdeen: University of Aberdeen [online], pages 85-88. Available from: https://knowingfromtheinside.org/files/unfinished.pdf Japan blues: ao, ai, midori, aizome, ao-ja-shin. CLARKE, J. 2017 This document was downloaded from https://openair.rgu.ac.uk IRON ORE JAPAN BLUES interchangeable. Ao is a good example; in Jan Peter Laurens Loovers Ao, Ai, Midori, Aizome, Ao-ja-shin Japan, ao is the colour of grass and leaves, Jen Clarke and traffic lights and the colour of the sky. … but folk in the housen, as the People of the It can be blue and green, or blue or green. It Hills call them, must be ruled by Cold Iron. These impressions of blue have come from can also be an-almost black, if it is a horse’s Folk in housen are born on the near side reflecting on visitors responses to my use of coat, or be used to imply something pale, of Cold Iron – there’s iron in every man’s the colour blue during a residency in Japan green, unripe, unready, unpalatable. The house, isn’t there? They handle Cold Iron that focussed on making and exhibiting 24 boundaries are not the same, but more than every day of their lives, and their fortune’s 24-hour cyanotype photograms. The exhibi- this, to think about blue in Japan, I also made or spoilt by Cold Iron in some shape or tion was arranged as an act of remembrance, think of green. -

PAINTING BEYOND the PHOTO (P-16) Laura Spector Intermediate / Advanced Monday Evening, 6:30 - 9:30 PM January 22, 29 and February 5, 12 4-Day Workshop
PAINTING BEYOND THE PHOTO (P-16) Laura Spector Intermediate / Advanced Monday evening, 6:30 - 9:30 PM January 22, 29 and February 5, 12 4-Day Workshop MATERIALS LIST Acrylic Paint: Please use Open Acrylics so they stay workable throughout class. Golden and Liquitex both make "Open" versions of their paint. If you already have heavy-bodied paint, you may want to contact the manufacturer to ask what medium they would recommend to add to their paint to make it "open". You will need a cool and warm of each primary color. 1. Titanium White (PW 6) 2. Zinc White (PW 4) 3. Pyrrole Red Light (PR 255) 4. Quinacridone Crimson (PR 206 / PR 202) 5. C.P. Cadmium Yellow Primrose (PY 35) 6. Hansa Yellow Medium (PY 73) 7. Yellow Ochre (PY 43) 8. Ultramarine Blue (PB 29) 9. Cerulean Blue (PB 36:1) 10. Burnt Sienna (PBr 7) 11. Burnt Umber (PBr 7) 12. Raw Umber (PBr 7) 13. Dioxazine Purple (PV 23) 14. Prussian Blue (PB15:1 / PV23 / Pbk9) 15. Cadmium Orange (PO 20) (This will likely be the most expensive color and used on most dark skin tones) *Optional - You can purchase SATIN Glazing Medium to extend your paints or use OPEN paints. Water Cup (Glass jar is helpful) Spray bottle (the kind for spraying house plants) Bag of WHITE rags (Southland Hardware - or any other hardware store has these) Art League School – Winter 2018 Oil Paint: *I have included the color index code for each pigment. (Ex: PY34). If you choose to use your favorite brand, please go by the color index code instead of the color name. -

FALCON® BEAM ARC Colour
FALCON® 1 BEAM 2 ARC colour 3,000W/7,000W 3,000 W I 7,000 W MADE IN GERMANY Powder-coated aluminium housing Weatherproof (IP44) 200,000 lumens output (7,000W) BEAM 2 ARC colour - Architectural Lighting ® 16 bit precision movement FALCON The FALCON® BEAM 2 ARC colour is perfect for large architectural and entertainment installations, including use as a searchlight or DATASHEET - long-throw washlight. Tilt movement 240 ° FALCON ® BEAM ARC colour, 3,000 W Pan movement 500 ° Dimensions 3,000 W Dimensions 7,000 W h: 1,320 mm I 52.0 inch h: 1,621 mm I 63,8 inch w: 601 mm I 23.7 inch w: 732 mm I 28.8 inch d: 730 mm I 28.7 inch d: 900 mm I 35.4 inch Pivoting angle: 240 ° Pivoting angle: 240 ° 686 mm I 27 inch w 490 mm I 19.3 inch inch mm I 22.3 566 h Working area inch inch h inch Working area 243mm 9.6 inch mm I 41.5 816 mm I 32.1 inch mm I 41.5 mm I 32.5 1,055 inch 825 1,055 d 706 mm I 27.8 inch w mm I 1.8 d 45 Technical subjects to alterations. Technical FALCON® BEAM 2 ARC colour FALCON® BEAM 2 ARC colour specifications 3,000 W 7,000 W Lamp 2 Type OSRAM XBO® 3,000 W OSRAM Xstage® 7,000 W Colour temperature 6,000 K Colour changer system Colour changer Gel scroller with up to 18 single colours Gel scroller with up to 13 single colours Reflector Nickel with high precision electro-shaped Nickel with high precision electro-shaped Material rhodium/dichroic coating rhodium/dichroic coating Diameter 320 mm / 12.6 inch 460 mm / 18.1 inch Function Lamp on / off, hot strike, electronic dimmer, electronic / mechanical strobe, douser, zoom, Main functions