P Hytopl Tid Lankto Al Cree N Com Ek
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oja Representational Objects in Yewaland, Ogun State, Nigeria Ayedun Matthew Kolawole Ab
African Scholar VOL. 18 NO. 6 Publications & ISSN: 2110-2086 Research SEPTEMBER, 2020 International African Scholar Journal of Humanities and Social Sciences (JHSS-6) Study of Forms and Functions of Esu – Oja Representational Objects in Yewaland, Ogun State, Nigeria Ayedun Matthew Kolawole Fine and Applied Arts Department, School of Vocational and Technical Education, Tai Solarin College of Education, Omu – Ijebu Abstract The representational images such as pots, rattles, bracelets, stones, cutlasses, wooden combs, staffs, mortals, brooms, etc. are religious and its significance for the worshippers were that they had faith in it. The denominator in the worship of all gods and spirits everywhere is faith. The power of an image was believed to be more real than that of a living being. The Yoruba appear to be satisfied with the gods with whom they are in immediate touch because they believe that when the Orisa have been worshipped, they will transmit what is necessary to Olodumare. This paper examines the traditional practices of the people of Yewa and discovers why Esu is market appellate. It focuses on the classifications of the forms and functions of Esu-Oja representational objects in selected towns in Yewaland. Keywords: appellate, classifications, divinities, fragment, forms, images, libations, mystical powers, Olodumare, representational, sacred, sacrifices, shrine, spirit archetype, transmission and transformation. Introduction Yewa which was formally called there are issues of intra-regional “Egbado” is located on Nigeria’s border conflicts that call for urgent attention. with the Republic of Benin. Yewa claim The popular decision to change the common origin from Ile-Ife, Oyo, ketu, Egbado name according to Asiwaju and Benin. -

Analysis of Beef Consumption Pattern Among Rural Households in Yewa South Local Government Area of Ogun State, Nigeria
Journal of Sustainable Development in Africa (Volume 17, No.8, 2015) ISSN: 1520-5509 Clarion University of Pennsylvania, Clarion, Pennsylvania ANALYSIS OF BEEF CONSUMPTION PATTERN AMONG RURAL HOUSEHOLDS IN YEWA SOUTH LOCAL GOVERNMENT AREA OF OGUN STATE, NIGERIA Akerele, Ezekiel Olaoluwa; Ologbon, Olugbenga Adesoji Christopher; Otunaiya, Abiodun Olanrewaju; and Ambali, Isiaka Omotuyole Department of Agricultural Economics and Farm Management, College of Agricultural Sciences Olabisi Onabanjo University, Yewa Campus, Ayetoro, Ogun State ABSTRACT This study analysed the consumption pattern of beef among rural households in Yewa South Local Government Area of Ogun State, Nigeria. A two-stage simple random sampling technique was employed, and with the help of 120 well- structured questionnaires, data were collected from 120 rural households. The collected data were then subjected to both descriptive and econometrics statistics (regression analysis, Marginal Propensity To Consume (MPC), price elasticity of beef). The results indicated that the highest age of the consumers was within the age bracket of 30-39 years. 65% of the population was married. Secondary education takes the dominance with 49.2% having it. Their major occupation was petty trading (45%). The results showed that beef price and monthly expenditure on food items negatively affected beef consumption in the area, while beef preference, fish price, beef availability, total monthly income and major occupation positively affected beef consumption. Price elasticity of beef was found out to be -0.90006. The Marginal Propensity Consume of beef was 0.0017484. The identified major constraint to beef consumption in the area was low availability of beef. It is therefore recommended that large scale beef cattle rearing should be encouraged which will help to increase the production of safe beef for consumption. -

Land Accessibility Characteristics Among Migrants in Yewa North Local Government Area of Ogun State, Nigeria
Asian Research Journal of Arts & Social Sciences 2(1): 1-12, 2017; Article no.ARJASS.30086 SCIENCEDOMAIN international www.sciencedomain.org Land Accessibility Characteristics among Migrants in Yewa North Local Government Area of Ogun State, Nigeria Gbenga John Oladehinde 1* , Kehinde Popoola 1, Afolabi Fatusin 2 and Gideon Adeyeni 1 1Department of Urban and Regional Planning, Obafemi Awolowo University, Ile-Ife, Osun State Nigeria. 2Department of Geography and Planning Sciences, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria. Authors’ contributions This work was carried out collaboratively by all authors. Author GJO designed the study and wrote the first draft of the manuscript. Authors KP and AF supervised the preparation of the first draft of the manuscript and managed the literature searches while author GA led and managed the data analyses. All authors read and approved the final manuscript. Article Information DOI: 10.9734/ARJASS/2017/30086 Editor(s): (1) Raffaela Giovagnoli, Pontifical Lateran University, Piazza San Giovanni in Laterano 4, Rome, Italy. (2) Sheying Chen, Social Policy and Administration, Pace University, New York, USA. Reviewers: (1) F. Famuyiwa, University of Lagos, Nigeria. (2) Lusugga Kironde, Ardhi University, Dar es Salaam, Tanzania. Complete Peer review History: http://www.sciencedomain.org/review-history/17570 Received 16 th October 2016 Accepted 14 th January 2017 Original Research Article st Published 21 January 2017 ABSTRACT Aim: The study investigated challenges of land accessibility among migrants in Yewa North Local Government Area of Ogun State, Nigeria. Methodology: Data were obtained through questionnaire administration on a Migrant household head. Multistage sampling technique was used for selection of 161 respondents for the study. -

African Concepts of Energy and Their Manifestations Through Art
AFRICAN CONCEPTS OF ENERGY AND THEIR MANIFESTATIONS THROUGH ART A thesis submitted to the College of the Arts of Kent State University in partial fulfillment of the requirements for the degree of Master of Arts by Renée B. Waite August, 2016 Thesis written by Renée B. Waite B.A., Ohio University, 2012 M.A., Kent State University, 2016 Approved by ____________________________________________________ Fred Smith, Ph.D., Advisor ____________________________________________________ Michael Loderstedt, M.F.A., Interim Director, School of Art ____________________________________________________ John R. Crawford-Spinelli, D.Ed., Dean, College of the Arts TABLE OF CONTENTS LIST OF FIGURES………………………………………….. iv ACKNOWLEDGMENTS …………………………………… vi CHAPTERS I. Introduction ………………………………………………… 1 II. Terms and Art ……………………………………………... 4 III. Myths of Origin …………………………………………. 11 IV. Social Structure …………………………………………. 20 V. Divination Arts …………………………………………... 30 VI. Women as Vessels of Energy …………………………… 42 VII. Conclusion ……………………………………….…...... 56 VIII. Images ………………………………………………… 60 IX. Bibliography …………………………………………….. 84 X. Further Reading ………………………………………….. 86 iii LIST OF FIGURES Figure 1: Porogun Quarter, Ijebu-Ode, Nigeria, 1992, Photograph by John Pemberton III http://africa.si.edu/exhibits/cosmos/models.html. ……………………………………… 60 Figure 2: Yoruba Ifa Divination Tapper (Iroke Ifa) Nigeria; Ivory. 12in, Baltimore Museum of Art http://www.artbma.org/. ……………………………………………… 61 Figure 3.; Yoruba Opon Ifa (Divination Tray), Nigerian; carved wood 3/4 x 12 7/8 x 16 in. Smith College Museum of Art, http://www.smith.edu/artmuseum/. ………………….. 62 Figure 4. Ifa Divination Vessel; Female Caryatid (Agere Ifa); Ivory, wood or coconut shell inlay. Nigeria, Guinea Coast The Metropolitan Museum of Art, http://www.metmuseum.org. ……………………… 63 Figure 5. Beaded Crown of a Yoruba King. Nigerian; L.15 (crown), L.15 (fringe) in. -
1 Geography and Society
Cambridge University Press 978-1-107-06460-7 — The Yoruba from Prehistory to the Present Aribidesi Usman , Toyin Falola Excerpt More Information 1 1 Geography and Society Stories of Odud uwa’s arrival at Il e- Ife, and his children’s subsequent migration into new territories (Atanda 1980 :2; Johnson 1921 ), mark the beginning of Yoruba history studies, from Ajayi Crowther and Samuel Johnson onwards. One origin legend, claiming that the Y oruba had inhabited their territory from time immemorial, begins with Olod umare (God) sending Oduduwa from heaven to create the solid earth and the human race (Atanda 1980 :1– 2). In the legend, Oduduwa descends to earth on a long chain and lands at Ile- Ife, where he establishes solid ground and plants the i rst seed (Akintoye 2004 :4– 5). This tradition establishes Ile-Ife as the cradle of the Yoruba, and Oduduwa as the Yoruba’s progenitor, or i rst ancestor. Oduduwa was considered the founder of the i rst Yoruba kingdom, situated in Ile- Ife, beginning the Yoruba kingship. Another version of the origin myth, detailing later developments, claims that Oduduwa led the Yoruba to their present location after migrating from the east. The story claims that the migration was caused by political disturbances accompanying the expansion of Islam (Atanda 1980 ), but the exact location of the legend’s “east” is not def- inite. A third claim, divergent from the above traditions, asserts that Ile- Ife was already inhabited when Oduduwa arrived. This introduces the story of Agbo nmiregun, whom Oduduwa met at Ile-Ife (Atanda 1980 ). -

UC Irvine Journal for Learning Through the Arts
UC Irvine Journal for Learning through the Arts Title UNITY IN DIVERSITY: THE PRESERVED ART WORKS OF THE VARIED PEOPLES OF ABEOKUTA FROM 1830 TO DATE Permalink https://escholarship.org/uc/item/2fp9m1q6 Journal Journal for Learning through the Arts, 16(1) Authors Ifeta, Chris Funke Idowu, Olatunji Adenle, John et al. Publication Date 2020 DOI 10.21977/D916138973 eScholarship.org Powered by the California Digital Library University of California Unity in Diversity: Preserved Art Works of Abeokuta from 1830 to Date and Developmental Trends * Chris Funke Ifeta, **Bukola Odesiri Ochei, *John Adenle, ***Olatunji Idowu, *Adekunle Temu Ifeta * Tai Solarin University of Education, Ijagun, Ijebu-Ode, Ogun State, Nigeria. **Faculty of Law, University of Ibadan, Ibadan, Oyo State, Nigeria ** *University of Lagos, Lagos State Please address correspondence to funkeifeta @gmail.com additional contacts: [email protected] (Ochei); [email protected] (Adenle); [email protected] (Ifeta, A.) Abstract Much has been written on the history of Abeokuta and their artworks since their occupation of Abeokuta. Yoruba works of art are in museums and private collections abroad. Many museums in the Western part of Nigeria including the National Museum in Abeokuta also have works of art on display; however, much of these are not specific to Abeokuta. Writers on Abeokuta works of art include both foreign and Nigerian scholars. This study uses historical theory to study works of art collected and preserved on Abeokuta since inception of the Egba, Owu and Yewa (Egbado) occupation of the town and looks at implications for development in the 21st century. The study involved the collection of data from primary sources within Abeokuta in addition to secondary sources of information on varied works of art including Ifa and Ogboni paraphernalia. -
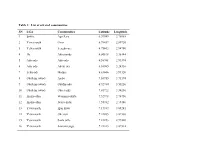
Table 1: List of Selected Communities SN LGA Communities Latitude
Table 1: List of selected communities SN LGA Communities Latitude Longitude 1 Ipokia Ago Sasa 6.59089 2.76065 2 Yewa-south Owo 6.78457 2.89720 3 Yewa-south Ireagbo-are 6.75602 2.94780 4 Ifo Akinsinnde 6.80818 3.16144 5 Ado-odo Ado-odo 6.58768 2.93374 6 Ado-odo Abebi-ota 6.68965 3.24330 7 Ijebu-ode Molipa 6.83606 3.91120 8 Obafemi-owode Ajebo 7.10955 3.71174 9 Obafemi-owode Odofin-odo 6.92744 3.55220 10 Obafemi-owode Oba-seriki 7.01712 3.34230 11 Imeko-afon Wasinmi-okuta 7.52948 2.76750 12 Imeko-afon Iwoye-ketu 7.55782 2.74486 13 Yewa-north Igan ikoto 7.15339 3.04281 14 Yewa-north Oke rori 7.24805 3.02368 15 Yewa-north Saala orile 7.21253 2.97420 16 Yewa-north Araromi joga 7.23323 3.02514 17 Ewekoro Abule Oko 6.86859 3.19430 18 Shagamu Ipoji 6.84440 3.65006 19 Shagamu Odelemo 6.74479 3.66392 20 Ikenne Irolu 6.90834 3.72447 21 Odogbolu Ikosa 6.83873 3.76291 22 Ijebu-east Itele 6.76299 4.06629 23 Ijebu-east Imobi 6.65920 4.17934 24 Ijebu north-east Atan 6.89712 4.00414 25 Abeokuta-south Ibon 7.15864 3.35519 26 Ijebu north Agric 6.93907 3.83253 27 Ijebu north Japara 6.97274 3.99278 28 Remo north Akaka 6.94053 3.71328 29 Odeda Alabata 7.31567 3.53351 30 Odeda Olodo 7.29659 3.60758 31 Abeokuta north Imala odo 7.32122 3.18115 32 Ogun water-side Abigi 6.48618 4.39408 33 Ogun water-side Iwopin 6.51054 4.16990 Table 2: Sex and age distribution of study participants SN LGA Sex (%) Age in years (%) Number Male Female <5yrs 5-15yrs 16-25yrs 26-40yrs 41-70yrs >70yrs Examined 1 Abeokuta north 87 28(32.2) 59(67.8) 7(8.0) 64(7.6) 9(10.3) 3(3.4) 4(4.6) -

PERCEIVED EFFECTS of QUARRY ACTIVITIES on COCOA PRODUCTION in YEWA NORTH LOCAL GOVERNMENT AREA of OGUN STATE, NIGERIA Olorode, W
Nigerian Journal of Rural Sociology Vol. 17, No. 2, 2017 2ERCEIVED EFFECTS OF HUARRY ACTIVITIES ON COCOA 2RODUCTION IN YEWA NORT. LOCAL GOVERNMENT AREA OF OGUN STATE, NIGERIA Olorode, I. A., Adejuwon, O. T. and Oyesola, O. 4. .epartment of Agricultural E0tension and Rural .evelopment, niversity of 2badan, 2badan, Nigeria Correspondence contact detailsA opeyemiadejuwonJgmail.com, tokunbooyesolaJgmail.com; 08069039762, 08023250458 A STRACT There had been an increase in the number of Duarries in Nigeria in the 19 th centuries. The activities of the Duarries have detrimental effects on the environment and more importantly on permanent crops; most especially cocoa which is a major commercial crop in the study area. Therefore, this study determined the perceived effects of Duarry activities on cocoa production in )ewa north local government area of Ogun State, Nigeria. A multi1 stage sampling procedure was used in selecting 120 cocoa farmers in the study area. .ata collected were analy6ed using descriptive statistical tools like mean, percentage, freDuency counts, standard deviation and inferential statistics like Chi1sDuare, PP5C and t1test. All (100L, of the farmers were married, with mean age of 53 a8.4years. 9owever, 42.5L of the cocoa farmers have a household si6e of between 5 and 6, while 86.7L of them had formal education with majority finishing secondary school. Environmental problems identified as being severe by respondents were soil erosion, air pollution and massive deforestation, while problems associated with Duarry activities were rock blasting, rock powdering and transportation. There is significant difference in the yield of cocoa production before and after the establishment of the cement industry in 2011 in the study area (tN120.851, pN0.0000,. -

Performance Analysis of Yam Marketing in Yewa Division of Ogun State, Nigeria
International Journal of Innovative Food, Nutrition & Sustainable Agriculture 7(4):10-21, Oct.-Dec., 2019 © SEAHI PUBLICATIONS, 2019 www.seahipaj.org ISSN: 2467-8481 Performance Analysis of Yam Marketing in Yewa Division of Ogun State, Nigeria 1Akerele, E. O. 2Yusuf, M. O., 3Solana O. I., 4Olugbemi M.T., & 5Yangomodou O.D. 1, 2Department of Agricultural Economics and Farm Management 3,4,5Department of Home Science and Hospitality Management Faculty of Agricultural Management and Rural Development College of Agricultural Sciences, Olabisi Onabanjo University Yewa Campus, Ayetoro Ogun State, Nigeria [email protected] 07031361498 ABSTRACT Globally, quest for empowerment have increased the need of investigation into the economic activities of marketers. This study focused performance analysis of yam marketing in Yewa Division of Ogun State, Nigeria with the specific objectives of describing the socio economic characteristics of the yam marketers; describe the cost and return structure of yam marketing; estimate the marketing margin and efficiency of yam and identify the problems militating against yam marketing in the study area. A two- stage sampling technique was used to select ten (10) markets. From each market, twelve (12) yam marketers were randomly selected and interviewed making a total of one hundred and twenty (120) sampled marketers for the study. Data collected were analyzed using descriptive statistics and budgetary analysis. Results obtained revealed that majority (95.8%) of the marketers were female with an average age of 41 years per marketer, married (85.8%), and illiterate with more than half of the respondents. Gross Margin (GM) and Net Income (NI) per month were N75,808.30 and N66,491.63 respectively implying that yam marketing was profitable in the study area. -

Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria
Spatial Distribution of Ascariasis, Trichuriasis and Hookworm Infections in Ogun State, Southwestern Nigeria Hammed Mogaji ( [email protected] ) Federal University Oye-Ekiti https://orcid.org/0000-0001-7330-2892 Gabriel Adewunmi Dedeke Federal University of Agriculture Abeokuta Babatunde Saheed Bada Federal University of Agriculture Abeokuta Samuel O. Bankole Federal University of Agriculture Abeokuta Adejuwon Adeniji Federal University of Agriculture Abeokuta Mariam Tobi Fagbenro Federal University of Agriculture Abeokuta Olaitan Olamide Omitola Federal University of Agriculture Abeokuta Akinola Stephen Oluwole SightSavers Nnayere Simon Odoemene Adeleke University Uwem Friday Ekpo Federal University of Agriculture Abeokuta Research article Keywords: Spatial Mapping, Distribution, Ascariasis, Trichuriasis, Hookworm, Ogun State, Nigeria Posted Date: July 29th, 2019 DOI: https://doi.org/10.21203/rs.2.12035/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/20 Abstract Background Ascariasis, Trichuriasis and Hookworm infections poses a considerable public health burden in Sub-Saharan Africa, and a sound understanding of their spatial distribution facilitates to better target control interventions. This study, therefore, assessed the prevalence of the trio, and mapped their spatial distribution in the 20 administrative regions of Ogun State, Nigeria. Methods Parasitological surveys were carried out in 1,499 households across 33 spatially selected communities. Fresh stool samples were collected from 1,027 consenting participants and processed using ether concentration method. Households were georeferenced using a GPS device while demographic data were obtained using a standardized form. Data were analysed using SPSS software and visualizations and plotting maps were made in ArcGIS software. Results Findings showed that 19 of the 20 regions were endemic for one or more kind of the three infections, with an aggregated prevalence of 17.2%. -
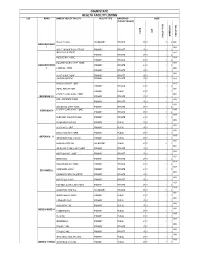
List of Coded Health Facilities in Ogun State.Pdf
OGUN STATE HEALTH FACILITY LISTING LGA WARD NAME OF HEALTH FACILITY FACILITY TYPE OWNERSHIP CODE (PUBLIC/ PRIVATE) LGA STATE OWNERSHIP FACILITY NO FACILITY FACILITY TYPE FACILITY Shallom Hospital SECONDARY PRIVATE 27 01 2 2 0001 IBEREKODO WARD 1 0002 UNCLE JOHN MEDICAL SERVICE PRIMARY PRIVATE 27 01 1 2 INSHA ALAHU EWEJE 0003 PRIMARY PRIVATE 27 01 1 2 MODUPE MAT. HOME 0004 PRIMARY PRIVATE 27 01 1 2 COLUMBIA CLINIC & MAT. HOME 0005 IBEREKODO WARD PRIMARY PRIVATE 27 01 1 2 2 LISABI MAT. HOME 0006 PRIMARY PRIVATE 27 01 1 2 0007 ALASELA MAT. HOME PRIMARY PRIVATE 27 01 1 2 JULIBAM HOSPITAL PRIMARY PRIVATE 27 01 1 2 0008 KING DAVIDS MAT. HOME 0009 PRIMARY PRIVATE 27 01 1 2 ROYAL HEALTH POST 0010 PRIMARY PUBLIC 27 01 1 1 CHARITY CLINIC & MAT. HOME 0011 IBEREKODO 111 PRIMARY PRIVATE 27 01 1 2 ADE – FAITH MAT. HOME 0012 PRIMARY PRIVATE 27 01 1 2 0013 GOD GRACE & MAT. HOME PRIMARY PRIVATE 27 01 1 2 IBEREKODO IV CHARITY CLINIC & MAT. HOME 0014 PRIMARY PRIVATE 27 01 1 2 0015 DURO MED. DIAGNOSTE LAB PRIMARY PRIVATE 27 01 1 2 0016 ELEGA HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0017 ALAFIA MAT. HOME PRIMARY PUBLIC 27 01 1 1 0018 OMOLAYAJO MAT. HOME PRIMARY PUBLIC 27 01 1 1 IBEREKODO V 0019 IBEREKODO HEALTH CLINIC PRIMARY PUBLIC 27 01 1 1 0020 GENERAL HOSPITAL SECONDARY PUBLIC 27 01 2 1 0021 IKE-OLUWA CLINIC & MAT. HOME PRIMARY PRIVATE 27 01 1 2 0022 MOPELOLA MAT. HOME PRIMARY PRIVATE 27 01 1 2 0023 BISTED MED PRIMARY PRIVATE 27 01 1 2 0024 TOLUWALASE MAT HOME PRIMARY PRIVATE 27 01 1 2 0025 LADE MAMO. -

Surface Runoff Simulation for Part of Yewa Basin
Predictions in Ungauged Basins: Promise and Progress (Proceedings of symposium S7 held during the 382 Seventh IAHS Scientific Assembly at Foz do Iguaçu, Brazil, April 2005). IAHS Publ. 303, 2006. Surface runoff simulation for part of Yewa basin OLUSEGUN ADEAGA1, LEKAN OYEBANDE1 & 2 CHRISTIAN DEPRAETERE 1 Department of Geography, University of Lagos, Nigeria [email protected] 2 Institut de Recherche pour le Développement (IRD), France Abstract PUB provides a scientific means of addressing water related prob- lems in a data-scarce basin like Yewa drainage basin in Nigeria. This study focuses on surface runoff simulation through the generation of geomorpho- logic instantaneous unit hydrographs using digital terrain modelling. The simulation is necessary, in order to provide relevant information needed towards water resources assessment within the basin. Key words digital elevation model; prediction of ungauged basin; synthetic hydrology; ungauged basin INTRODUCTION The unabated increase in water demand and its fluctuation constitute issues of concern in the planning and development of water resources, most especially in the area of water production and water utilization in the developing world (Shiklomanov, 1998). Thus, a search towards developing a sustainable water resources management scheme in any environment requires a thorough assessment of the water resources of such an environment. This is necessary, if a sound science-based policy on sustainable protection and use of available fresh water resources is to be attained for the growing population and their increasing water demands. Unfortunately, water resources assessment has been greatly affected by pressure of economic stringency through insufficient budget allocation and varied neglect of water resources assessment infrastructure.