Stibnite Sb2s3 C 2001-2005 Mineral Data Publishing, Version 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stibnite Gold Project
STIBNITE GOLD PROJECT PAYETTE & BOISE NATIONAL FORESTS INTERMOUNTAIN REGION August 22, 2018 STIBNITE MINING DISTRICT, PAYETTE NATIONAL FOREST, KRASSEL RANGER DISTRICT Regarding Locatable Minerals , 36 CFR 228, Subpart A: requires the Forest Service to: "Where conflicting interests Respond to a mining plan, evaluate that plan must be reconciled, the Consider requirements to minimize adverse effects to the extent feasible question shall always be Comply with applicable laws, regulations and answered from the stand- standards for environmental protection point of the greatest good of Assure appropriate reclamation Further respond by following National Environmental the greatest number in the Policy Act (NEPA) processes. long run." - James Wilson, Secretary Per 36 CFR 228.4, the Forest Service is required to take of Agriculture, 1905 in a letter to Gifford action to ensure that “operations are conducted so as, Pinchot, 1st Chief of the Forest Service. where feasible, to minimize adverse environmental impacts on National Forest surface resources. 1 Table of Contents Project Context STIBNITE GOLD Project Location 4 Mining History at Stibnite 5 PROJECT Who is Midas Gold 6 Stibnite Plan of Operations PAYETTE & BOISE NATIONAL Proposed Mining Plan of FOREST - INTERMOUNTAIN REGION Operations 7 Open Pit Mining 8 Anadromous Fish - Tunnel 9 Inventoried Roadless Areas - Why is this project of interest to many Access and Powerline 10 people and organizations? Facilities During Mining Operations 10 5 million recoverable ounces of gold x -

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Metacinnabar Hgs C 2001-2005 Mineral Data Publishing, Version 1
Metacinnabar HgS c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Cubic. Point Group: 43m. Commonly massive; rarely as small (to 1 mm) tetrahedral crystals having rough faces. Twinning: Common on {111}, forming lamellae in polished section. Physical Properties: Fracture: Subconchoidal. Tenacity: Brittle. Hardness = 3 VHN = n.d. D(meas.) = 7.65 D(calc.) = 7.63 Optical Properties: Opaque. Color: Grayish black; in polished section, grayish white. Streak: Black. Luster: Metallic. Pleochroism: Weak, rarely. Anisotropism: Very weak, rarely. R: (400) 28.4, (420) 27.6, (440) 26.8, (460) 26.3, (480) 25.9, (500) 25.6, (520) 25.4, (540) 25.2, (560) 25.1, (580) 25.0, (600) 24.9, (620) 24.9, (640) 24.8, (660) 24.7, (680) 24.7, (700) 24.7 Cell Data: Space Group: F 43m. a = 5.8717(5) Z = 4 X-ray Powder Pattern: Synthetic. 3.378 (100), 2.068 (55), 1.7644 (45), 2.926 (35), 1.3424 (12), 1.6891 (10), 1.3085 (10) Chemistry: (1) (2) (3) (4) Hg 79.73 67.45 81.33 86.22 Zn 4.23 3.10 Cd 11.72 Fe trace 0.2 Se 1.08 6.49 S 14.58 15.63 10.30 13.78 Total 99.62 98.10 98.12 100.00 (1) Guadalc´azar,Mexico; corresponds to (Hg0.85Zn0.14)Σ=0.99(S0.97Se0.03)Σ=1.00. (2) Uland area, Russia; corresponds to (Hg0.69Cd0.21Zn0.10Fe0.01)Σ=1.01S1.00. (3) San Onofre, Mexico; corresponds to Hg1.00(S0.80Se0.20)Σ=1.00. -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

A Deposit Model for Mississippi Valley-Type Lead-Zinc Ores
A Deposit Model for Mississippi Valley-Type Lead-Zinc Ores Chapter A of Mineral Deposit Models for Resource Assessment 2 cm Sample of spheroidal sphalerite with dendritic sphalerite, galena, and iron sulfides (pyrite plus marcasite) from the Pomorzany mine. Note the “up direction” is indicated by “snow-on-the-roof” texture of galena and Scientificsphalerite Investigations alnong colloform Report layers of2010–5070–A light-colored spahlerite. Hydrothermal sulfide clasts in the left center of the sample are encrusted by sphalerire and iron sulfides. Size of sample is 20x13 cm. Photo by David Leach. U.S. Department of the Interior U.S. Geological Survey COVER: Sample of spheroidal sphalerite with dendritic sphalerite, galena, and iron sulfides (pyrite plus mar- casite) from Pomorzany mine. Note the “up direction” is indicated by “snow-on-the-roof” texture of galena and sphalerite along colloform layers of light-colored sphalerite. Hydrothermal sulfide clasts in the left center of the sample are encrusted by sphalerite and iron sulfides. Size of sample is 20x13 centimeters. (Photograph by David L. Leach, U.S. Geological Survey.) A Deposit Model for Mississippi Valley- Type Lead-Zinc Ores By David L. Leach, Ryan D. Taylor, David L. Fey, Sharon F. Diehl, and Richard W. Saltus Chapter A of Mineral Deposit Models for Resource Assessment Scientific Investigations Report 2010–5070–A U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. -

Pyrite's Evil Twin Marcasite and Pyrite Are Two Common Minerals. Both Fes2 Chemically, Making Them Polymorphs. Polym
Marcasite - Pyrite's Evil Twin Marcasite and pyrite are two common minerals. Both FeS2 chemically, making them polymorphs. Polymorphs are minerals with the same chemical composition but different crystal structures. Diamond and graphite are polymorphs, both minerals being pure carbon. In diamond and graphite the different arrangement of carbon atoms gives these two minerals of very different physical properties. Pyrite and marcasite, on the other hand, have almost identical physical properties, making them tough to tell from each other. Let's go through their properties. Both are metallic and pale yellow to brassy yellow. Both can tarnish and be iridescent. Both are 6-6.5 on the Mohs' hardness scale. Neither have a particularly prominent cleavage, although marcasite does have one that occasionally shows up. Both have densities of about 5 grams per cubic centimeter (pyrite is a bit denser, but not enough to be detectible without delicate measurements). They can even be found together in the same rock. Fortunately these minerals often show good outer crystal shapes that are quite different. Pyrite crystals are generally equant, and dominated by cubes, octahedrons and 12-sided pyritohedrons. Marcasite crystals are usually rectangular (tabular) with wedge-shaped ends and tend to form in star shaped, radiating or cockscomb groups. Marcasite is also much more restricted in occurrence than pyrite, forming only in low temperature, near surface, very acidic environments. It is found in some ore deposits, in sediments formed under somewhat stagnant conditions and as ground water precipitates in rocks such as in limestone and shale. Although pyrite can also be found in many of these same environments, the crystal shapes are diagnostic. -

Ity of Minerais with the Pyrite, Marcasite
STRUCTURALSTABI!.ITY OF MINERAISWITH THE PYRITE, MARCASITE,ARSENOPYRITE AND L6ILINGITESTRUCTURES ERNEST H. NICKEL M,ineral Sc'iencesD'i,v,is,ion, M,ines Branch, Departrnentof Energy, M.i,nesand, Resowrces, 6id Booth Street,Ottawa, Canad,a AssrRAcr The structural stabilities of the disulphides, diarsenides and sulpharsenides of iron, cobalt and nickel are explained on thi basis of ligand field theory. ihe structural stabilities can be correlated with the number of non-bonding d elecirons of the metal atom in the structure, and can.be explained by the tenden"yif th" .ornpourrd" to form structures in which maximum electron spin-pairing takes place. The pyrite,structure, which is favoured-by -!tub with six or more non-bonding d electrons, and which includes pyrite, cattierite, vaesite, cobaltite gur"aormt"i i, characterized by metal-sulphur oclahedra joined at corneis, with no apparintlnteraction""a between the d electrons of neighbouring metal atoms. The otiei structures are all characterized by shared octahedral edgesalong one direction, so that the metal atoms are brought into.relatively close proximlty. In-the marcasite structure, which includes marcasite and rammelsbergite, both with six non-bonding d electrons, the metal atoms repel each other because of completely filled tzs levek. In dre arsenopyrite structure, whichjncludes arsenopyrite and siffiorite, both oi which havefrve iJ eleciions that do noi participate.in metal-sulphur londing, pairs of metals are drawn together to permit spin- pairing of the odd electrons. In ldllingite, in which the iron atom islssumed io have iour non-bonding-d electrons, the d orbitals in.the a crystallographic direction are emptied, permitting close iron-iron approachesin this direciion, r"-wull as complete of the electrons in the two remaining ,2sorbitals. -

Descriptive Mineralogy of Pugh Quarry, Northwestern Ohio: Sphalerite1
Copyright © 1978 Ohio Acad. Sci. 0030-0950/78/0005-027211.50/0 DESCRIPTIVE MINERALOGY OF PUGH QUARRY, NORTHWESTERN OHIO: SPHALERITE1 DAVID F. PARR2 and LUKE L. Y. CHANG, Department of Geology, Miami University, Oxford, OH 45056 Abstract. The Devonian rocks at Pugh Quarry have three distinct types of sphalerite (banded massive, spheroidal, and tiny euhedral). Occurrence of the banded massive sphalerite is restricted to the mineral zone, predominantly as blebs in marcasite. The color of banded sphalerite ranged from nearly colorless to various hues of yellow. The replacement of banded sphalerite by marcasite was observed. The spheroidal sphalerite occurred in association with marcasite of euhedral habit. The spherules were small, the largest no greater than 1 mm across. Where present in great pro- fusion, the sphalerite spherules merged together forming botryoidal surfaces. The euhedral sphalerite occurred in the voids of the sponge-like and stromatolitic dolostone below the mineral zone, and in a layer of soft laminated mud associated with the dolostone. The euhedral sphalerite was predominantly red-brown, and no crystals larger than 1 mm in maximum dimension were observed. OHIO J. SCI. 78(5): 272, 1978 Sphalerite was found in cavities in all RELATION TO HOST ROCK parts of Pugh Quarry. Along with mar- Where sphalerite occurred between casite (Parr and Chang 1977), it com- the bands of marcasite and the dolostone prised nearly all of the sulfide mineraliza- host rock, replacement relationships could tion. Sphalerite occurred in three dis- be observed between the two. Dolomite tinct types. The banded sphalreite par- rhombs were commonly observed to be tially replaced by marcasite was the most engulfed in the banded sphalerite. -

Clinocervantite, ~-Sb204, the Natural Monoclinic Polymorph of Cervantite from the Cetine Mine, Siena, Italy
Eur. J. Mineral. 1999,11,95-100 Clinocervantite, ~-Sb204, the natural monoclinic polymorph of cervantite from the Cetine mine, Siena, Italy RICCARDO BASSO 1, GABRIELLA LUCCHETTI I, LIVIa ZEFIRO 1 and ANDREA PALENZONA 2 IDipartimento di Scienze delia Terra dell'Universita, Corso Europa 26, 1-]6] 32 Genova, Ita]y e-mail: minera]@dister.unige.it 2Dipartimento di Chi mica e Chimica industria]e dell'Universita, Via Dodecaneso 3],1-]6]46 Genova, Ita]y Abstract: Clinocervantite occurs at the Cetine di Cotorniano mine associated with valentinite, tripuhyite, bindheimite and rosiaite. Clinocervantite, appearing generally as aggregates of single prisms elongated along [001] or twinned on {100}, is co]ourless, transparent, with vitreous lustre, biaxial, with the lowest measured ] ] refractive index a,' = .72 and the highest one y' = 2. O. The strongest lines in the powder pattern are dill = 3.244 A and d311 = 2.877 A. The crystal structure, space group C2Ie with a = 12.061(1) A, b = 4.836(1) A, ] e = 5.383( I) A, ~= 04.60( 4)" and Z = 4, has been refined to R = 0.020, confirming the new mineral to be the natural analogue of the synthetic ~-Sb204 already known. The structures of clinocervantite and cervantite may be regarded as built up by stacking layers of nearly identical structure and composition accounting for both polytypism in the Sb204 compound and twinning of the clinocervantite crystals. Key-words: clinocervantite, crystal-structure refinement, cervantite, twinning. Introduction Occurrence, physical properties and chemical composition During the study of rosiaite (Basso et al., 1996), an associated new mineral was found in materia] Clinocervantite occurs in litt]e cavities of a rock from the Cetine mine, central Tuscany, Italy. -

H20 , a New Mineral from Pereta, Tuscany, Italy, and Two Other Localities
Coquandite, Sb608(SO4).H20 , a new mineral from Pereta, Tuscany, Italy, and two other localities C. SABELL1 CNR--Centro di studio per la Mincralogia e la Geochimica dei sedimenti, Via La Pira 4, 50121 Firenze, Italy P. ORLANDI Dipartimento di Scienze della Terra, Universitfi di Pisa, Via S. Maria 53, 56100 Pisa, Italy AND G. VEZZAL1NI Istituto di Mineralogia e Petrologia, Universit~ di Modena, Via S. Eufemia 19, 41100 Modena, Italy Abstract Coaquandite, a new antimony oxy-sulphate hydrate, occurs as spheroidal knobs of silky fibres or, rarely, as tiny transparent colourless lamellar crystals on stibnite at the Pereta mine, Tuscany, Italy; it is associated with klebelsbergite, peretaite, valentinite, sulfur, gypsum, stibiconite, and senarmontite. Coquandite is triclinic P1,.... with a 11.434(7), b 29.77(4), c 11.314(4) A,~ ~ 91.07(7) o , 13 119.24(3) ~ y 92.82(1) o . It has a cell volume of 3352(5)A~ 3 with Z = 12 and a calculated density of 5.78 gcm --J . The crystals, elongated along [001] and flattened on {010}, display polysynthetic twinning with (010) as the twin plane. Optically, they are biaxial (+) with z ~ c, 2V >> 60 ~ n = 2.08(5). The strongest lines of the X-ray powder pattern are [d in A, (I), (hkl)] 14.84(50)(020), 9.27(41)(111, 110), 6.81(67)130, 3.304(93)(090), 3.092(100)(330). Coquandite has also been found at the Cetine mine, Tuscany, Italy, and at the Lucky Knock mine, Tonasket, Okanogan County, Washington, USA. 22 microprobe chemical analyses (elemental microanalysis for H) gave Sb203 88.91, SO3 8.35, CaO 0.04, Na20 0.03, H20 1.43, total 98.76 wt.%, corresponding to the empirical formula (Sb + S = 7) Sbs.98Ca0.01Nao.0107.96(SO4)~.02.0"78H20, and to the idealised formula Sb608(SO4).H20. -
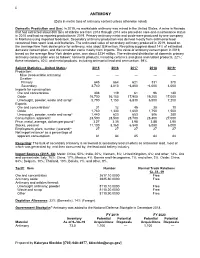
Antimony Data Sheet
22 ANTIMONY (Data in metric tons of antimony content unless otherwise noted) Domestic Production and Use: In 2019, no marketable antimony was mined in the United States. A mine in Nevada that had extracted about 800 tons of stibnite ore from 2013 through 2014 was placed on care-and-maintenance status in 2015 and had no reported production in 2019. Primary antimony metal and oxide were produced by one company in Montana using imported feedstock. Secondary antimony production was derived mostly from antimonial lead recovered from spent lead-acid batteries. The estimated value of secondary antimony produced in 2019, based on the average New York dealer price for antimony, was about $34 million. Recycling supplied about 14% of estimated domestic consumption, and the remainder came mostly from imports. The value of antimony consumption in 2019, based on the average New York dealer price, was about $234 million. The estimated distribution of domestic primary antimony consumption was as follows: nonmetal products, including ceramics and glass and rubber products, 22%; flame retardants, 40%; and metal products, including antimonial lead and ammunition, 39%. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine (recoverable antimony) — — — — — Smelter: Primary 645 664 621 331 370 Secondary 3,740 3,810 e3,800 e4,000 4,000 Imports for consumption: Ore and concentrates 308 119 61 96 140 Oxide 16,700 16,100 17,900 19,200 17,000 Unwrought, powder, waste and scrap1 5,790 7,150 6,830 6,500 7,200 Exports: Ore and concentrates1 31 -

New Mineral Names*
American Mineralogist, Volume 66, pages 1274-1280, 1981 NEW MINERAL NAMES* MICHAEL FLEISCHER AND LOUIS J. CABRI Cyanophillite* ar~ 3.350(50)(110), 3.:208(50)(020), 3.080 (80)(111), 2.781(100) (221,111), 2.750(70)(112), 1.721 (60). Kurt Walenta (1981) Cyanophillite, a new mineral from the Clara ~olor1ess to white, luster vitreous. Cleavages {010}, {OOI}, Mine, near Oberwolfach, Central Black Forest. Chem. der {OIl} good, not easily observed. Hardness about 5. Optically Erde, 40, 195-200 (in German). biaxial positive, ns ex= 1.713, /3 = 1.730, )' = 1.748, 2V +88° (89° Analyses gave CuO 36.3, 32.5; Ah03 8.5, -; Sb203 36.5, 38.3; calc.). Material with Zn:Mg = 1:1 is biaxial, neg., ns. ex= 1.689, H20 19.8; sum 101.1%, corresponding to 10CuO . 2Ah03 . 3Sb2 /3 = 1.707, )' = 1.727, 2V ~ 85°. 03 . 25H20. The mineral is dissolved readily by cold 1:1 HCI, The mineral occurs as coatings and small crystals, largest partly dissolved by 1: 1 HN03. Loss of weight when heated (%) dimension about 1 mm; on prosperite, adamite, and austinite 110° 3.4, 150° 9.5, 200° 19.8%. At 250° the mineral is decomposed from Tsumeb, Namibia. Forms observed {010}, {001}, {Oil}, also and turns black. {IOO}very small. X-ray study shows the mineral to be orthorhombic, space The name is for Robert I. Gait, Curator of Mineralogy, Royal group Pmmb, a = 11.82, b = 10.80, c = 9.64A, Z = 1, D 3.10 Ontario Museum, Toronto. Type material is at the Royal Ontario meas., 3.12 calc.