Endemic and Alien Vascular Plant Diversity in the Small Mediterranean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flora Mediterranea 26
FLORA MEDITERRANEA 26 Published under the auspices of OPTIMA by the Herbarium Mediterraneum Panormitanum Palermo – 2016 FLORA MEDITERRANEA Edited on behalf of the International Foundation pro Herbario Mediterraneo by Francesco M. Raimondo, Werner Greuter & Gianniantonio Domina Editorial board G. Domina (Palermo), F. Garbari (Pisa), W. Greuter (Berlin), S. L. Jury (Reading), G. Kamari (Patras), P. Mazzola (Palermo), S. Pignatti (Roma), F. M. Raimondo (Palermo), C. Salmeri (Palermo), B. Valdés (Sevilla), G. Venturella (Palermo). Advisory Committee P. V. Arrigoni (Firenze) P. Küpfer (Neuchatel) H. M. Burdet (Genève) J. Mathez (Montpellier) A. Carapezza (Palermo) G. Moggi (Firenze) C. D. K. Cook (Zurich) E. Nardi (Firenze) R. Courtecuisse (Lille) P. L. Nimis (Trieste) V. Demoulin (Liège) D. Phitos (Patras) F. Ehrendorfer (Wien) L. Poldini (Trieste) M. Erben (Munchen) R. M. Ros Espín (Murcia) G. Giaccone (Catania) A. Strid (Copenhagen) V. H. Heywood (Reading) B. Zimmer (Berlin) Editorial Office Editorial assistance: A. M. Mannino Editorial secretariat: V. Spadaro & P. Campisi Layout & Tecnical editing: E. Di Gristina & F. La Sorte Design: V. Magro & L. C. Raimondo Redazione di "Flora Mediterranea" Herbarium Mediterraneum Panormitanum, Università di Palermo Via Lincoln, 2 I-90133 Palermo, Italy [email protected] Printed by Luxograph s.r.l., Piazza Bartolomeo da Messina, 2/E - Palermo Registration at Tribunale di Palermo, no. 27 of 12 July 1991 ISSN: 1120-4052 printed, 2240-4538 online DOI: 10.7320/FlMedit26.001 Copyright © by International Foundation pro Herbario Mediterraneo, Palermo Contents V. Hugonnot & L. Chavoutier: A modern record of one of the rarest European mosses, Ptychomitrium incurvum (Ptychomitriaceae), in Eastern Pyrenees, France . 5 P. Chène, M. -

Mencan Rock Garden Etu
Bulletin of the mencan Rock Garden etu VOL. 41 SUMMER 1983 NO. 3 THE BULLETIN Editor . Laura Louise Foster, Falls Village, Conn. 06031 Assistant Editor... Harry Dewey, 4605 Brandon Lane, Beltsville, Md. 20705 Contributing Editors . Roy Davidson, Anita Kistler, H. Lincoln Foster, Owen Pearce, H.N. Porter Layout Designer . Buffy Parker Advertising Manager... Anita Kistler, 1421 Ship Rd., West Chester, Pa. 19380 CONTENTS VOL. 41 NO. 3 SUMMER 1983 Southeastern Sun and Sand — Pam Harper 105 Some Poppy Kin — Geoffrey Charlesworth Ill Plant Hunting in Sichuan, China: Part II — Carla Teune 114 Insect Encounters of a Pleasant Kind — Sharon J. Collman 123 Some Native Clematis — John J. Wurdack 125 Draba Polytricha: Hardy (?) Alpine Jewel — Dr. Daniel C. Weaver 129 Award Winners - 1983: Award of Merit, Betty Ann Mech; LePiniec Award, Robert Putnam 131 Getting Acquainted With North Carolina Flora — Sandra Ladendorf 135 Book Reviews: Rock Garden and Alpine Plants by Raymond Foster; The Rhododendron Species, Vol. I, Lepidotes by H. H. Davidian; Jewels of the Plains - Wildflowers of the Great Plains Grasslands and Hills by Claude A. Barr 137 Claude Barr's Plants — Norman C. Deno 141 Of Cabbages and Kings 142 Cover Picture — Papaver miyabeanum — Carol Ann Kearns Princeton, New Jersey Published quarterly by the AMERICAN ROCK GARDEN SOCIETY, incorporated under the laws of the State of New Jersey. You are invited to join. Annual dues (Bulletin included), to be submitted in U.S. Funds or International Money Order, are: General Membership, SI5.00 (includes domestic or foreign, single or joint - 2 at same address to receive 1 Bulletin, 1 Seed List); Patron, $50.00; Life Member, $250.00. -

The Down Rare Plant Register of Scarce & Threatened Vascular Plants
Vascular Plant Register County Down County Down Scarce, Rare & Extinct Vascular Plant Register and Checklist of Species Graham Day & Paul Hackney Record editor: Graham Day Authors of species accounts: Graham Day and Paul Hackney General editor: Julia Nunn 2008 These records have been selected from the database held by the Centre for Environmental Data and Recording at the Ulster Museum. The database comprises all known county Down records. The records that form the basis for this work were made by botanists, most of whom were amateur and some of whom were professional, employed by government departments or undertaking environmental impact assessments. This publication is intended to be of assistance to conservation and planning organisations and authorities, district and local councils and interested members of the public. Cover design by Fiona Maitland Cover photographs: Mourne Mountains from Murlough National Nature Reserve © Julia Nunn Hyoscyamus niger © Graham Day Spiranthes romanzoffiana © Graham Day Gentianella campestris © Graham Day MAGNI Publication no. 016 © National Museums & Galleries of Northern Ireland 1 Vascular Plant Register County Down 2 Vascular Plant Register County Down CONTENTS Preface 5 Introduction 7 Conservation legislation categories 7 The species accounts 10 Key to abbreviations used in the text and the records 11 Contact details 12 Acknowledgements 12 Species accounts for scarce, rare and extinct vascular plants 13 Casual species 161 Checklist of taxa from county Down 166 Publications relevant to the flora of county Down 180 Index 182 3 Vascular Plant Register County Down 4 Vascular Plant Register County Down PREFACE County Down is distinguished among Irish counties by its relatively diverse and interesting flora, as a consequence of its range of habitats and long coastline. -

Phylogeography of Western Mediterranean Cymbalaria
www.nature.com/scientificreports OPEN Phylogeography of western Mediterranean Cymbalaria (Plantaginaceae) reveals two Received: 5 December 2017 Accepted: 19 November 2018 independent long-distance Published: xx xx xxxx dispersals and entails new taxonomic circumscriptions Pau Carnicero 1,2, Peter Schönswetter2, Pere Fraga Arguimbau3, Núria Garcia-Jacas4, Llorenç Sáez1,5 & Mercè Galbany-Casals1 The Balearic Islands, Corsica and Sardinia (BCS) constitute biodiversity hotspots in the western Mediterranean Basin. Oligocene connections and long distance dispersal events have been suggested to cause presence of BCS shared endemic species. One of them is Cymbalaria aequitriloba, which, together with three additional species, constitute a polyploid clade endemic to BCS. Combining amplifed fragment length polymorphism (AFLP) fngerprinting, plastid DNA sequences and morphometrics, we inferred the phylogeography of the group and evaluated the species’ current taxonomic circumscriptions. Based on morphometric and AFLP data we propose a new circumscription for C. fragilis to additionally comprise a group of populations with intermediate morphological characters previously included in C. aequitriloba. Consequently, we suggest to change the IUCN category of C. fragilis from critically endangered (CR) to near threatened (NT). Both morphology and AFLP data support the current taxonomy of the single island endemics C. hepaticifolia and C. muelleri. The four species had a common origin in Corsica-Sardinia, and two long-distance dispersal events to the Balearic Islands were inferred. Finally, plastid DNA data suggest that interspecifc gene fow took place where two species co-occur. Islands in the Mediterranean Basin harbour both high species diversity and endemism. For instance, from the around 5000 islands scattered in the Mediterranean Sea1 all the largest ones (i.e. -

Arenaria Balearica (Caryophyllaceae) Javier Bobo-Pinilla, Noemí López-González & Julio Peñas De Giles
Supplementary file 1 to: Conservation of genetic diversity in Mediterranean endemic species: Arenaria balearica (Caryophyllaceae) Javier Bobo-Pinilla, Noemí López-González & Julio Peñas de Giles Plant Ecology and Evolution 153(3), 2020 Supplementary file 1 – Probabilities of loss of 474 rare AFLP alleles when all populations of Arenaria balearica are considered as one single management unit and preferred sampling area. Npop, number of populations where each AFLP allele was found; Nind, number of individuals where the alleles were found; geographical regions: MAJ = Majorca, COR = Corsica, SAR = Sardinia; Lo, observed probability of loss of the allele; Le, expected probability of loss of the allele; PSA, preferred sampling area. Npop Nind PSA Lo Le Average Rare allele All MAJ COR SAR MAJ COR SAR frequency B004 3 1 1 1 1 1 1 0.4632 0.0027 0.12 B012 4 0 3 1 0 9 1 COR 0.0849 0.0000 0.27 B017 2 0 0 2 0 0 3 SAR 0.5410 0.0009 0.14 B024 2 1 0 1 1 0 2 SAR 0.4358 0.0001 0.19 B030 3 2 0 1 3 0 1 MAJ 0.3674 0.0005 0.15 B031 1 0 0 1 0 0 1 SAR 0.8789 0.0514 0.06 B033 3 1 1 1 2 2 1 0.2967 0.0001 0.18 B044 4 0 1 3 0 1 11 SAR 0.0558 0.0000 0.30 B047 4 2 1 1 2 1 1 MAJ 0.4200 0.0068 0.10 B053 3 1 0 2 1 0 2 SAR 0.5057 0.0054 0.11 B070 2 0 0 2 0 0 2 SAR 0.6204 0.0041 0.11 B071 4 2 0 2 4 0 2 SAR 0.2357 0.0002 0.17 B076 4 0 2 2 0 2 2 0.3849 0.0041 0.11 B080 4 2 1 1 3 1 1 MAJ 0.3128 0.0013 0.14 B083 1 1 0 0 1 0 0 MAJ 0.8100 0.0079 0.10 B084 3 1 0 2 1 0 11 SAR 0.0429 0.0000 0.41 B094 3 0 1 2 0 1 5 SAR 0.2621 0.0000 0.20 B097 1 1 0 0 1 0 0 MAJ 0.7656 0.0021 0.13 -

Downloaded from the Worldclim Website ( Accessed on 15 January 2021) Under Current Climatic Conditions (1970– 2000)
diversity Article Reusing Old and Producing New Data Is Useful for Species Delimitation in the Taxonomically Controversial Iberian Endemic Pair Petrocoptis montsicciana/ P. pardoi (Caryophyllaceae) Neus Nualart 1, Sonia Herrando-Moraira 1, Eduardo Cires 2,3 , Moisès Guardiola 4 , Emilio Laguna 5 , David Pérez-Prieto 1, Llorenç Sáez 4 and Jordi López-Pujol 1,* 1 Botanic Institute of Barcelona (IBB, CSIC-Ajuntament de Barcelona), Passeig del Migdia, s/n, 08038 Barcelona, Spain; [email protected] (N.N.); [email protected] (S.H.-M.); [email protected] (D.P.-P.) 2 Departamento de Biología de Organismos y Sistemas, Universidad de Oviedo, Área de Botánica. C/Catedrático Rodrigo Uría s/n, 33071 Oviedo, Spain; [email protected] 3 Instituto de Recursos Naturales y Ordenación del Territorio (INDUROT), Campus de Mieres, C/Gonzalo Gutiérrez Quirós s/n, 33600 Mieres, Spain 4 Systematics and Evolution of Vascular Plants (UAB)—Associated Unit to CSIC, Departament de Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain; [email protected] (M.G.); [email protected] (L.S.) 5 Generalitat Valenciana, Servicio de Vida Silvestre—CIEF (Centro para la Investigación y Experimentación Forestal), Avda. Comarques del País Valencià, 114, 46930 València, Spain; [email protected] Citation: Nualart, N.; * Correspondence: [email protected] Herrando-Moraira, S.; Cires, E.; Guardiola, M.; Laguna, E.; Abstract: Petrocoptis montsicciana and P. pardoi are two Iberian endemic taxa of Caryophyllaceae family Pérez-Prieto, D.; Sáez, L.; López-Pujol, with an unclear taxonomic delimitation, being variously treated as independent species, subspecies J. -

Flowering Plants List
The Islay Natural History Trust's R* Japanese Red-cedar Cryptomeria japonica C Alder Alnus glutinosa L* Grey Alder A.incana Checklist of the Wild Flowers of Islay and Jura This list includes all species reliably reported on Islay and Jura. The distribution and status of many species is poorly known and all records are valuable. Please send them, with localities and dates, to the Islay Wildlife Information Centre, Port Charlotte, Isle of Islay, PA48 7TX. The following status indications are necessarily only approximate and are based on the number of 10 km squares where the plant occurs, out of the 14 covering Islay. C=widespread, usually common, 8 or more squares, L=less widespread, but can be common suitable habitat, 3-7 squares. R=rare or very local, 1 or 2 squares only. *= introduced or escaped. +=needs confirming. J=Jura only. O=old records, pre-1950. LYCOPODIACEAE CUPRESSACEAE R* Hornbeam Carpinus betulus L Fir Clubmoss Huperzia selago R* Gowen Cypress Cupressus goveniana C Hazel Corylus avellana J Stag’s-horn Clubmoss Lycopodium clavatum L* Lawson’s Cypress Chamaecyparis lawsoniana CHENOPODIACEAE R Alpine Clubmoss Diphasiastrum alpinum R* Western Red-cedar Thuja plicata C Fat-hen Chenopodium album SELAGINELLACEAE C Juniper Juniperus communis commmunis C Spear-leaved Orache Atriplex prostrata C Lesser Clubmoss Selaginella selaginoides J J.communis nana C Babington’s Orache A.glabriuscula ISOETACEAE ARAUCARIACEAE C Common Orache A.patula L Quillwort Isoetes lacustris R* Monkey-puzzle Araucaria araucana L Frosted Orache A.laciniata -

Balearic Vegetation
Plant Formations in the Balearic BioProvince Peter Martin Rhind Balearic Evergreen Oak Forests Like much of the Mediterranean, the natural plant formation, is evergreen oak forest dominated by Quercus ilex (holm oak) although today Pinus halepensis (aleppo pine) is probably the most common tree on the islands. Other tree species include Arbutus unedo strawberry tree, while the shrub layer comprises Asparagus acutifolius, Daphne gnidium (Mediterranean mezereon), Erica arborea (tree heather), Phillyrea latifolia and the near endemic Rhamnus ludovici-salvatoris (Rhamnaceae). The ground layer includes another species, Cyclamen balearicum (Myrsinaceae), that was originally thought to be endemic, but this species has since been recorded in the south of France. Balearic Olive Woods The dominant tree here is Olea europaea var. silvestris (wild olive) and Ceratonia silique (carob). Typical shrubs include Cneorum tricoccon, Euphorbia dendroides (tree spurge), Ephedra fragilis (joint-pine) and Chamaerops humulis (dwarf fan palm) may also be present. Ground layer species typically include Arum pictum, Asparagus albus and A. stipularis. Balearic Rosemary Garrigue The main shrubs in this formation include Rosmarius officinalis (rosemary) and Erica multiflora. Rosemary can be found from sea level to the top of the highest mountain, but the endemic variety palaui is confined to the mountains. Other important shrubs include Anthyllus cytisoides, Globularia alypum, Lavendula dentata and the endemic Genista lucida (Fabaceae). Characteristic herbaceous species include Gladiolus illyricus and various ‘insect’ orchids such as Ophrys tenthredinifera sawfly orchid and O. bombylifera bumblebee orchid. Endemics include Smilax aspera var. balearica (Liliaceae). Balearic Mountain Garrigue Most Balearic endemics occur in this zone. The endemic shrubs Hypericum balearicum (Hypericaceae) or Teucrium subspinosum (Lamiaceae) are two of the most conspicuous species. -
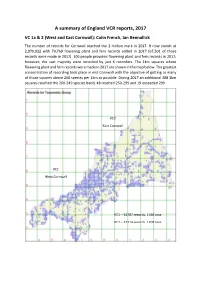
A Summary of England VCR Reports, 2017
A summary of England VCR reports, 2017 VC 1a & 2 (West and East Cornwall): Colin French, Ian Bennallick The number of records for Cornwall reached the 2 million mark in 2017. It now stands at 2,070,922 with 79,769 flowering plant and fern records added in 2017 (67,501 of those records were made in 2017). 106 people provided flowering plant and fern records in 2017; however, the vast majority were recorded by just 6 recorders. The 1km squares where flowering plant and fern records were made in 2017 are shown in the map below. The greatest concentration of recording took place in mid Cornwall with the objective of getting as many of those squares above 200 species per 1km as possible. During 2017 an additional 308 1km squares reached the 200-249 species band, 48 reached 250-299 and 19 exceeded 299. VC2 East Cornwall VC1 West Cornwall VC1 – 34787 records, 1166 taxa VC2 – 32714 records, 1209 taxa Number of species of flowering plants and ferns per 1km recorded since 1999 – up to 31st December 2017 Continued good progress was made in 2017 towards the ongoing monad survey which will result in the publication of the New Flora of Cornwall atlas. West Cornwall (VC1) is largely complete. 22 of the 3963 1km squares remain unvisited and of the 1909 1km squares that have less than 200 species recorded (see table below) it is estimated that 800-900 1km squares can be significantly improved. It should be noted that a large part of central VC2 consists of Bodmin Moor (circa 200 km2) where the maximum number of species per 1km square is close to 100 and many coastal squares only support low numbers of species. -

Phylogeography of Arenaria Balearica L. (Caryophyllaceae): Evolutionary History of a Disjunct Endemic from the Western Mediterranean Continental Islands
Phylogeography of Arenaria balearica L. (Caryophyllaceae): evolutionary history of a disjunct endemic from the Western Mediterranean continental islands Javier Bobo-Pinilla1,2, Sara B. Barrios de León1, Jaume Seguí Colomar3, Giuseppe Fenu4, Gianluigi Bacchetta5, Julio Peñas de Giles6 and María Montserrat Martínez-Ortega1,2 1 Department of Botany, University of Salamanca, Salamanca, Spain 2 Biobanco de ADN Vegetal, Banco Nacional de ADN, University of Salamanca, Salamanca, Spain 3 Department of Terrestrial Ecology, Instituto Mediterráneo de Estudios Avanzados (IMEDEA), Esporles, Spain 4 Dipartimento di Biologia Ambientale, University of Roma ``La Sapienza'', Roma, Italy 5 Centro Conservazione Biodiversità (CCB), Dipartimento di Scienze della Vita e dell'Ambiente, University of Cagliari, Cagliari, Italy 6 Department of Botany, University of Granada, Granada, Spain ABSTRACT Although it has been traditionally accepted that Arenaria balearica (Caryophyllaceae) could be a relict Tertiary plant species, this has never been experimentally tested. Nor have the palaeohistorical reasons underlying the highly fragmented distribution of the species in the Western Mediterranean region been investigated. We have analysed AFLP data (213) and plastid DNA sequences (226) from a total of 250 plants from 29 populations sampled throughout the entire distribution range of the species in Majorca, Corsica, Sardinia, and the Tuscan Archipelago. The AFLP data analyses indicate very low geographic structure and population differentiation. Based on plastid DNA data, six alternative phylogeographic hypotheses were tested using Approximate Bayesian Computation (ABC). These analyses revealed ancient area fragmentation as the most probable scenario, which is in accordance with the star-like topology of the Submitted 16 March 2016 Accepted 27 September 2016 parsimony network that suggests a pattern of long term survival and subsequent in situ Published 3 November 2016 differentiation. -
Aberystwyth University the Reappearance of Lobelia Urens From
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aberystwyth Research Portal Aberystwyth University The reappearance of Lobelia urens from soil seed bank at a site in South Devon Smith, R. E. N. Published in: Watsonia Publication date: 2002 Citation for published version (APA): Smith, R. E. N. (2002). The reappearance of Lobelia urens from soil seed bank at a site in South Devon. Watsonia, 107-112. http://hdl.handle.net/2160/4028 General rights Copyright and moral rights for the publications made accessible in the Aberystwyth Research Portal (the Institutional Repository) are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the Aberystwyth Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the Aberystwyth Research Portal Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. tel: +44 1970 62 2400 email: [email protected] Download date: 09. Jul. 2020 Index to Watsonia vols. 1-25 (1949-2005) by Chris Boon Abbott, P. P., 1991, Rev. of Flora of the East Riding of Yorkshire (by E. Crackles with R. Arnett (ed.)), 18, 323-324 Abbott, P. -
BSBI News No. 91
2 Administration and Important Addresses 11 ADMINISTRATION AND IMPORTANT ADDRESSES 11 PRESIDENT Mr Richard Pryce Trevethin, School Road, Pwll, Llanelli, Carmarthenshire, SA15 4AL Tel. & Fax: 01554775847; e-mail: PryceEco@ao!.com HON. GENERAL SECRETARY (General Enquiries) Miss Ailsa Burns 3 Rosliston Road, Stapenhill, Burton-upon-Trent, Staffordshire DE15 9RJ Te!.: 01283-568136; e-mail: BSBIHonGenSec@ao!.com HON. TREASURER (All financial matters except SUbscriptions) Mr Michael Braithwaite 19 Buccleuch Street, Hawick, Roxburghshire, TD9 OHL Te!.: 01450-372267; Fax: 01450-373591 HON. EDITOR (BSBI NEWS) Mr Gwynn Ellis 41 Marlborough Road, Roath, Cardiff CF23 5BU Te!. & Fax: 029-2049-6042; e-mail: BSBINewsEditor@ao!.com or [email protected] MEMBERSHIP SECRETARY (payment of Subs and changes of address) Mr Michael Walpole 68 Outwoods Road, Loughborough, Leics. LEll 3LY (Please quote membership number ou all correspondence; it is on the address label on your mailings, and in the List of Members in YearBook 2001 and 2002) Te!': 01509-215598; e-mail: mike.walpole@dia!.pipex.com HON. FIELD SECRETARY (Enquiries on Field Meetings) Mrs Jane Croft 12 Spaldwick Road, Stow Longa, Huntingdon, Cambs. PE28 OTL E-mail: [email protected] BSBI PROJECT MANAGER Mr David Pearman The Old Rectory, Frome St Quintin, Dorchester, Dorset DT2 OHF Te!.: 01935-83702; e-mail: DPearman4@ao!.com BSBI CO-ORDINATOR Mr Alex Lockton 66 North Street, Shrewsbury, Shropshire, SYl 2JL Te!. & Fax: 01743 343789; Mobile: 0585 700368; e-mail: [email protected] BSBI VOLUNTEER OFFICER Mr Pete Selby 12 Sedgwick Road, Bishopstoke, Eastleigh, Hampshire S050 6FH . Te!. 02380 644368; e-mail: [email protected] WATSONIA RECEIVING EDITOR Mr Martin N.