Biomonitoring of Environmental Status and Trends
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 Alabama Department of Environmental Management
ALABAMA DEPARTMENT OF ENVIRONMENTAL MANAGEMENT WATER DIVISION - WATER QUALITY PROGRAM CHAPTER 335-6-10 WATER QUALITY CRITERIA TABLE OF CONTENTS 335-6-10-.01 Purpose 335-6-10-.02 Definitions 335-6-10-.03 Water Use Classifications 335-6-10-.04 Antidegradation Policy 335-6-10-.05 General Conditions Applicable to All Water Quality Criteria 335-6-10-.06 Minimum Conditions Applicable to All State Waters 335-6-10-.07 Toxic Pollutant Criteria Applicable to State Waters 335-6-10-.08 Waste Treatment Requirements 335-6-10-.09 Specific Water Quality Criteria 335-6-10-.10 Special Designations 335-6-10-.11 Water Quality Criteria Applicable to Specific Lakes 335-6-10-.12 Implementation of the Antidegradation Policy 335-6-10-.01 Purpose. (1) Title 22, Section 22-22-1 et seq., Code of Alabama 1975, includes as its purpose "... to conserve the waters of the State and to protect, maintain and improve the quality thereof for public water supplies, for the propagation of wildlife, fish and aquatic life and for domestic, agricultural, industrial, recreational and other legitimate beneficial uses; to provide for the prevention, abatement and control of new or existing water pollution; and to cooperate with other agencies of the State, agencies of other states and the federal government in carrying out these objectives." (2) Water quality criteria, covering all legitimate water uses, provide the tools and means for determining the manner in which waters of the State may be best utilized, provide a guide for determining waste treatment requirements, and provide the basis for standards of quality for State waters and portions thereof. -
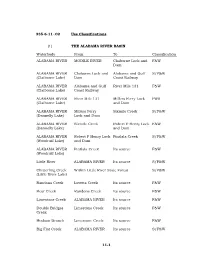
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

Chapter 335-6-11 Water Use Classifications for Interstate and Intrastate Waters
Environmental Management Chapter 335-6-11 DEPARTMENT OF ENVIRONMENTAL MANAGEMENT WATER DIVISION - WATER QUALITY PROGRAM ADMINISTRATIVE CODE CHAPTER 335-6-11 WATER USE CLASSIFICATIONS FOR INTERSTATE AND INTRASTATE WATERS TABLE OF CONTENTS 335-6-11-.01 The Use Classification System 335-6-11-.02 Use Classifications 335-6-11-.01 The Use Classification System. (1) Use classifications utilized by the State of Alabama are as follows: Outstanding Alabama Water ................... OAW Public Water Supply ......................... PWS Swimming and Other Whole Body Shellfish Harvesting ........................ SH Fish and Wildlife ........................... F&W Limited Warmwater Fishery ................... LWF Agricultural and Industrial Water Supply ................................ A&I (2) Use classifications apply water quality criteria adopted for particular uses based on existing utilization, uses reasonably expected in the future, and those uses not now possible because of correctable pollution but which could be made if the effects of pollution were controlled or eliminated. Of necessity, the assignment of use classifications must take into consideration the physical capability of waters to meet certain uses. (3) Those use classifications presently included in the standards are reviewed informally by the Department's staff as the need arises, and the entire standards package, to include the use classifications, receives a formal review at least once every three years. Efforts currently underway through local 201 planning projects will provide additional technical data on certain waterbodies in the State, information on treatment alternatives, and applicability of various management techniques, which, when available, will hopefully lead to new decisions regarding use classifications. Of particular interest are those segments which are currently classified for any usage which has an associated Supp. -

July 3-9, 2011
Department of Natural Resources Wildlife Resources Division Law Enforcement Section Field Operations Weekly Report July 3-9, 2011 This report is a broad sampling of events that have taken place in the past week, but does not include all actions taken by the Law Enforcement Section. Region I- Calhoun (Northwest) FLOYD COUNTY On Sunday June 26th, Cpl. Shawn Elmore was working along the Etowah River in Rome and overheard radio traffic from Rome PD about a vehicle getting broken into at the Etowah River boat ramp. Cpl. Elmore responded to the boat ramp and assisted Rome PD officer. Cpl. Elmore located one of the suspects hiding in the woods and the other suspect came out of the river. Three juvenile suspects were detained and charged with entering an auto and criminal damage to property by Rome PD. On June 26th, Cpl. Shawn Elmore was at the Heritage Park boat ramp on the Coosa River in Rome when he was approached by a parent of four teenagers that were caught in a storm on the Etowah River. Cpl. Elmore went to get his patrol vessel and while enroute to the Etowah River boat ramp the four teens called the parents and advised they had gotten out of the river safely and were waiting on a ride on Turner Chapel Rd. On the night of June 26th, Cpl. Shawn Elmore received a call from Floyd E911 about 3 adults and a 3 year-old child stranded on the Etowah River. Cpl. Elmore responded to the scene and assisted Rome/Floyd Fire with a search of the river and the four were located and safely returned to their vehicle. -
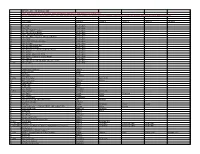
IMPORTANT INFORMATION: Lakes with an Asterisk * Do Not Have Depth Information and Appear with Improvised Contour Lines County Information Is for Reference Only
IMPORTANT INFORMATION: Lakes with an asterisk * do not have depth information and appear with improvised contour lines County information is for reference only. Your lake will not be split up by county. The whole lake will be shown unless specified next to name ex (Northern Section) (Near Follette) etc. LAKE NAME COUNTY COUNTY COUNTY COUNTY COUNTY GL Great Lakes Great Lakes GL Lake Erie Great Lakes GL Lake Erie (Port of Toledo) Great Lakes GL Lake Erie (Western Basin) Great Lakes GL Lake Erie with Lake Ontario Great Lakes GL Lake Erie (Eastern Section, Mentor to Buffalo) Great Lakes GL Lake Huron Great Lakes GL Lake Huron (w West Lake Erie) Great Lakes GL Lake Michigan Great Lakes GL Lake Michigan (Northeast) Great Lakes GL Lake Michigan (South) Great Lakes GL Lake Michigan (w Lake Erie and Lake Huron) Great Lakes GL Lake Ontario Great Lakes GL Lake Ontario (Rochester Area) Great Lakes GL Lake Ontario (Stoney Pt to Wolf Island) Great Lakes GL Lake Superior Great Lakes GL Lake Superior (w Lake Michigan and Lake Huron) Great Lakes GL (MI) Lake St Clair Great Lakes AL Cedar Creek Reservoir Franklin AL Deerwood Lake Shelby AL Dog River Mobile AL Gantt Lake Covington AL (GA) Goat Rock Lake * Lee Harris (GA) AL Guntersville Lake Marshall Jackson AL Highland Lake * Blount AL Inland Lake * Blount AL Jordan Lake Elmore AL Lake Gantt * Covington AL (FL) Lake Jackson * Covington Walton (FL) AL Lake Martin Coosa Elmore Tallapoosa AL Lake Mitchell Chilton Coosa AL Lake Tuscaloosa Tuscaloosa AL Lake Wedowee (RL Harris Reservoir) Clay Randolph AL Lake -

Depth Information Not Available for Lakes Marked with an Asterisk (*)
DEPTH INFORMATION NOT AVAILABLE FOR LAKES MARKED WITH AN ASTERISK (*) LAKE NAME COUNTY COUNTY COUNTY COUNTY GL Great Lakes Great Lakes GL Lake Erie Great Lakes GL Lake Erie (Port of Toledo) Great Lakes GL Lake Erie (Western Basin) Great Lakes GL Lake Huron Great Lakes GL Lake Huron (w West Lake Erie) Great Lakes GL Lake Michigan (Northeast) Great Lakes GL Lake Michigan (South) Great Lakes GL Lake Michigan (w Lake Erie and Lake Huron) Great Lakes GL Lake Ontario Great Lakes GL Lake Ontario (Rochester Area) Great Lakes GL Lake Ontario (Stoney Pt to Wolf Island) Great Lakes GL Lake Superior Great Lakes GL Lake Superior (w Lake Michigan and Lake Huron) Great Lakes AL Baldwin County Coast Baldwin AL Cedar Creek Reservoir Franklin AL Dog River * Mobile AL Goat Rock Lake * Chambers Lee Harris (GA) Troup (GA) AL Guntersville Lake Marshall Jackson AL Highland Lake * Blount AL Inland Lake * Blount AL Lake Gantt * Covington AL Lake Jackson * Covington Walton (FL) AL Lake Jordan Elmore Coosa Chilton AL Lake Martin Coosa Elmore Tallapoosa AL Lake Mitchell Chilton Coosa AL Lake Tuscaloosa Tuscaloosa AL Lake Wedowee Clay Cleburne Randolph AL Lay Lake Shelby Talladega Chilton Coosa AL Lay Lake and Mitchell Lake Shelby Talladega Chilton Coosa AL Lewis Smith Lake Cullman Walker Winston AL Lewis Smith Lake * Cullman Walker Winston AL Little Lagoon Baldwin AL Logan Martin Lake Saint Clair Talladega AL Mobile Bay Baldwin Mobile Washington AL Mud Creek * Franklin AL Ono Island Baldwin AL Open Pond * Covington AL Orange Beach East Baldwin AL Oyster Bay Baldwin AL Perdido Bay Baldwin Escambia (FL) AL Pickwick Lake Colbert Lauderdale Tishomingo (MS) Hardin (TN) AL Shelby Lakes Baldwin AL Walter F. -

Economic Analysis of Critical Habitat Designation for the Fat Threeridge, Shinyrayed Pocketbook, Gulf Moccasinshell, Ochlockonee
ECONOMIC ANALYSIS OF CRITICAL HABITAT DESIGNATION FOR THE FAT THREERIDGE, SHINYRAYED POCKETBOOK, GULF MOCCASINSHELL, OCHLOCKONEE MOCCASINSHELL, OVAL PIGTOE, CHIPOLA SLABSHELL, AND PURPLE BANKCLIMBER Draft Final Report | September 12, 2007 prepared for: U.S. Fish and Wildlife Service 4401 N. Fairfax Drive Arlington, VA 22203 prepared by: Industrial Economics, Incorporated 2067 Massachusetts Avenue Cambridge, MA 02140 Draft – September 12, 2007 TABLE OF CONTENTS EXECUTIVE SUMMARY ES-1T SECTION 1 INTRODUCTION AND FRAMEWORK FOR ANALYSIS 1-1 1.1 Purpose of the Economic Analysis 1-1 1.2 Background 1-2 1.3 Regulatory Alternatives 1-9 1.4 Threats to the Species and Habitat 1-9 1.5 Approach to Estimating Economic Effects 1-9 1.6 Scope of the Analysis 1-13 1.7 Analytic Time Frame 1-16 1.8 Information Sources 1-17 1.9 Structure of Report 1-18 SECTION 2 POTENTIAL CHANGES IN WATER USE AND MANAGEMENT FOR CONSERVATION OF THE SEVEN MUSSELS 2-1 2.1 Summary of Methods for Estimation of Economic Impacts Associated with Flow- Related Conservation Measures 2-2 2.2 Water Use in Proposed Critical Habitat Areas 2-3 2.3 Potential Changes in Water Use in the Flint River Basin 2-5 2.4 Potential Changes in Water Management in the Apalachicola River Complex (Unit 8) 2-10 2.5 Potential Changes in Water Management in the Santa Fe River Complex (Unit 11) 2-22 SECTION 3 POTENTIAL ECONOMIC IMPACTS RELATED TO CHANGES IN WATER USE AND MANAGEMENT 3-1 3.1 Summary 3-6 3.2 Potential Economic Impacts Related to Agricultural Water Uses 3-7 3.3 Potential Economic Impacts Related -

Paddle Georgia 2014 Fall Float on the Flint Oct
Albany Allemande– Paddle Georgia 2014 Fall Float on the Flint Oct. 10—Flint River Distance: 14 miles Starting Elevation: 190 feet Lat: 31.6022°N Lon: 84.1381°W Ending Elevation: 151 feet Lat: 31.4388°N Lon: 84.1423°W Restroom Facilities: Mile 0 Flint River Hydro Dam Mile 4.7 Radium Springs Boat Ramp Mile 14 Mitchell County Landing Points of Interest: Mile 0.2—Muckafoonee Creek—A short distance up this creek on river right is the 1906 dam that created “Lake Worth.” Today, the dam serves as an overflow spillway for the larger dam on the Flint. This oddly named waterway is a combination of two even more oddly named creeks: Kinchafoonee and Muckalee creeks. The Creek Indian word Kinchafoonee is believed to have meant “Mortar Nutshells” while Muckalee, recorded the Indian Agent Benjamin Hawkins, meant “Pour on me.” While this is the site of one of the first hydro-power dams in South Georgia, the Georgia General Assembly had earlier established laws specifically protecting Kinchafoonee Creek from obstructions that would prevent fish passage. The 1876 law prohibited the construction of any “dam, trap, net, seine or other device for catching fish, unless the channel is left for six feet.” There was, of course, a major loophole in the law: “nothing herein contained shall be so construed to prevent the erection of dams for milling and manufacturing purposes,” and thus a dam came to be built on Kinchafoonee. These lyrical names still echo through the region’s culture. The Kinchafoonee Cowboys is a well-known hony-tonk band from the area and Leesburg’s Luke Bryan, included an ode to fishing, boating, four-wheeling and drinking called “Muckalee Creek Water” on his 2011 album Tailgates and Tanlines. -

2018 Integrated 305(B)
2018 Integrated 305(b)/303(d) List - Streams Reach Name/ID Reach Location/County River Basin/ Assessment/ Cause/ Size/Unit Category/ Notes Use Data Provider Source Priority Alex Creek Mason Cowpen Branch to Altamaha Not Supporting DO 3 4a TMDL completed DO 2002. Altamaha River GAR030701060503 Wayne Fishing 1,55,10 NP Miles Altamaha River Confluence of Oconee and Altamaha Supporting 72 1 TMDL completed TWR 2002. Ocmulgee Rivers to ITT Rayonier GAR030701060401 Appling, Wayne, Jeff Davis Fishing 1,55 Miles Altamaha River ITT Rayonier to Penholoway Altamaha Assessment 20 3 TMDL completed TWR 2002. More data need to Creek Pending be collected and evaluated before it can be determined whether the designated use of Fishing is being met. GAR030701060402 Wayne Fishing 10,55 Miles Altamaha River Penholoway Creek to Butler Altamaha Supporting 27 1 River GAR030701060501 Wayne, Glynn, McIntosh Fishing 1,55 Miles Beards Creek Chapel Creek to Spring Branch Altamaha Not Supporting Bio F 7 4a TMDL completed Bio F 2017. GAR030701060308 Tattnall, Long Fishing 4 NP Miles Beards Creek Spring Branch to Altamaha Altamaha Not Supporting Bio F 11 4a TMDL completed Bio F in 2012. River GAR030701060301 Tattnall Fishing 1,55,10,4 NP, UR Miles Big Cedar Creek Griffith Branch to Little Cedar Altamaha Assessment 5 3 This site has a narrative rank of fair for Creek Pending macroinvertebrates. Waters with a narrative rank of fair will remain in Category 3 until EPD completes the reevaluation of the metrics used to assess macroinvertebrate data. GAR030701070108 Washington Fishing 59 Miles Big Cedar Creek Little Cedar Creek to Ohoopee Altamaha Not Supporting DO, FC 3 4a TMDLs completed DO 2002 & FC (2002 & 2007). -

FLINT RIVER TRAILS Master Plan and Implementation Strategy
FLINT RIVER TRAILS Master Plan and Implementation Strategy Prepared for Prepared by Albany/ Dougherty County, KAIZENCOLLABORATIVE Georgia October 10, 2016 Albany/ Dougherty County Greenway Master Plan October 10, 2016 Prepared for Albany/ Dougherty County, Georgia Prepared by: KAIZENCOLLABORATIVE 1668 Belle Isle Circle, NE | Atlanta, GA | 30329 [Page Intentionally Left Blank] Table of Contents Executive Summary 1 Introduction 1 4 Design Standards 67 2 Methodology 2 Introduction 68 4.1 Flint River Trails Logo 68 2.1 Planning Process 2 4.2 Trail Signage Standards 70 2.2 Steering Committee 2 4.3 Trail Amenities 71 2.3 Data Collection and Analysis 3 4.4 Bike Parking 73 2.4 Greenway Trail Development 4 4.5 Construction Details and Standards 74 2.5 Public Meetings 4 2.6 Types of Trails 5 5 Maintenance, Operations, and Recommendations 80 3 Flint River Trails Master Plan 9 5.1 Management Plan 81 Overview 10 5.2 Operation Responsibilities 81 3.1 Trail Segments 10 5.3 Trail Security 82 3.2 How to Determine Costs 10 5.4 Land Development Regulations 83 3.3 Trail Segment #1: Albany/Sasser Trail to Riverfront Trail 13 5.5 ‘Next Steps’ Checklist 83 3.4 Trail Segment #2: Chehaw Connection 29 3.5 Trail Segment #3: Albany State University to Paul Eames Sports Complex 37 Public Survey Results 84 3.6 Trail Segment #4: Albany State University Appendix: Steering Committee Members 86 To Radium Springs 48 3.7 Trail Segment #5: Downtown to Boy Scout Property 55 3.8 Implementation Strategy 63 Executive Summary World class trail systems connect a variety of destinations and experiences, offering something for all types of trail users. -

Unimpaired Flow Data Report Surface Water Availability Modeling and Technical Analysis for Statewide Water Management Plan
Unimpaired Flow Data Report Surface Water Availability Modeling and Technical Analysis for Statewide Water Management Plan Prepared for: Georgia Department of Natural Resources Prepared by: ARCADIS U.S., Inc. 2849 Paces Ferry Road Suite 400 Atlanta Georgia 30339 Tel 770.431.8666 Fax 770.435.2666 Our Ref.: GA063853/Rpt 2514 Date: April 12, 2010 This document is intended only for the use of the individual or entity for which it was prepared and may contain information that is privileged, confidential and exempt from disclosure under applicable law. Any dissemination, distribution or copying of this document is strictly prohibited. Table of Contents Executive Summary 1 1. Introduction 4 1.1 Need for Unimpaired Flows 4 1.2 Basic and Planning Node Selection 4 2. General Procedures for Unimpaired Flow Development 10 2.1 General Description of Unimpaired Flow Process 10 2.1.1 Reach Cases 11 2.2 Data Inventory and Management 12 2.2.1 Water Use Data 12 2.2.2 Streamflow Data 13 2.2.3 Routing Model Parameterization 13 2.2.4 Reservoir Physical and Operational Data 14 2.2.5 Reservoir Meteorological Data 14 2.2.5.1 Precipitation 15 2.2.5.2 Evaporation Time Series Development 25 2.2.5.3 Reservoir Runoff Coefficient Selection 27 2.2.6 Data Management Tools 32 2.2.7 Data Management Nomenclature 34 2.3 Reservoir Effects Calculation 40 2.3.1 Holdouts 41 2.3.2 Net Evaporation 41 2.3.3 Net Reservoir Effects 42 2.4 Flow Record Filling 42 2.4.1 Statistical Methods 44 2.4.2 Mean Flow Ratio 45 2.4.3 Drainage Area Ratio 46 c:\documents and settings\administrator\desktop\surface -

Two-Dimensional Flood-Inundation Model of the Flint River at Albany, Georgia
Two-Dimensional Flood-Inundation Model of the Flint River at Albany, Georgia Scientific Investigations Report 2007–5107 In cooperation with Albany, Georgia, and Dougherty County, Georgia U.S. Department of the Interior U.S. Geological Survey Cover photograph: Broad Avenue bridge over the Flint River at Albany, Georgia, March 2005 Photograph by Jonathan W. Musser, U.S. Geological Survey Two-Dimensional Flood-Inundation Model of the Flint River at Albany, Georgia By Jonathan W. Musser and Thomas R. Dyar In cooperation with Albany, Georgia, and Dougherty County, Georgia Scientific Investigations Report 2007–5107 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Musser, Jonathan W., and Dyar, Thomas R., 2007, Two-dimensional flood-inundation model of the Flint River at Albany, Georgia: Atlanta, Georgia, U.S. Geological Survey Scientific Investigations Report 2007-5107, 49 p., Web-only publication at http://pubs.usgs.gov/sir/2007/5107 iii Contents Abstract .......................................................................................................................................................