5.01.551 Granulocyte Colony-Stimulating Factor (G-CSF) Use in Adult
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Overview Colony Stimulating Factors
Therapeutic Class Overview Colony Stimulating Factors Therapeutic Class Overview/Summary: This review will focus on the granulocyte colony stimulating factors (G-CSFs) and granulocyte- macrophage colony stimulating factors (GM-CSFs).1-5 Colony-stimulating factors (CSFs) fall under the naturally occurring glycoprotein cytokines, one of the main groups of immunomodulators.6 In general, these proteins are vital to the proliferation and differentiation of hematopoietic progenitor cells.6-8 The G- CSFs commercially available in the United States include pegfilgrastim (Neulasta®), filgrastim (Neupogen®), filgrastim-sndz (Zarxio®), and tbo-filgrastim (Granix®). While filgrastim-sndz and tbo- filgrastim are the same recombinant human G-CSF as filgrastim, only filgrastim-sndz is considered a biosimilar drug as it was approved through the biosimilar pathway. At the time tbo-filgrastim was approved, a regulatory pathway for biosimilar drugs had not yet been established in the United States and tbo-filgrastim was filed under its own Biologic License Application.9 Only one GM-CSF is currently available, sargramostim (Leukine). These agents are Food and Drug Administration (FDA)-approved for a variety of conditions relating to neutropenia or for the collection of hematopoietic progenitor cells for collection by leukapheresis.1-5 Due to the pathway taken, tbo-filgrastim does not share all of the same indications as filgrastim and these two products are not interchangeable. It is important to note that although filgrastim-sndz is a biosimilar product, and it was approved with all the same indications as filgrastim at the time, filgrastim has since received FDA-approval for an additional indication that filgrastim-sndz does not have, to increase survival in patients with acute exposure to myelosuppressive doses of radiation.1-3A complete list of indications for each agent can be found in Table 1. -

The Evolution of Biosimilars in Oncology, with a Focus on Trastuzumab
EVOLUTIONPERSPECTIVES OF BIOSIMILARS IN INONCOLOGY, ONCOLOGY Nixon et al. The evolution of biosimilars in oncology, with a focus on trastuzumab †‡ N.A. Nixon MD,* M.B. Hannouf PhD, and S. Verma MD* ABSTRACT Cancer therapy has evolved significantly with increased adoption of biologic agents (“biologics”). That evolution is especially true for her2 (human epidermal growth factor receptor-2)–positive breast cancer with the introduction of trastuzumab, a monoclonal antibody against the her2 receptor, which, in combination with chemotherapy, significantly improves survival in both metastatic and early disease. Although the efficacy of biologics is undeniable, their expense is a significant contributor to the increasing cost of cancer care. Across disease sites and indications, biosimilar agents are rapidly being developed with the goal of offering cost-effective alternatives to biologics. Biosimilars are pharmaceuticals whose molecular shape, efficacy, and safety are similar, but not identical, to those of the original product. Although these agents hold the potential to improve patient access, complexities in their production, evaluation, cost, and clinical application have raised questions among experts. Here, we review the landscape of biosimilar agents in oncology, with a focus on trastu- zumab biosimilars. We discuss important considerations that must be made as these agents are introduced into routine cancer care. Key Words Biosimilars, trastuzumab, Herceptin, value Curr Oncol. 2018 Jun;25(S1):S171-S179 www.current-oncology.com INTRODUCTION alternatives to biologics. Although these agents have the potential to improve patient access, complexities in their In recent years, cancer treatment has been revolutionized production, evaluation, and clinical application have raised by the introduction of biologic agents (“biologics”). -

Biopharmaceutical Notes
An Overview of the BioPharmaceutical Products and Market By Paul DiMarco, Vice President, Global Commercial Program, BioSpectra Inc. Table of Contents INTRODUCTION: ........................................................................................................................................ 2 History ....................................................................................................................................................... 2 Background information ........................................................................................................................... 3 Defining biological products ..................................................................................................................... 4 Small vs. Large Molecule Regulation and Registration in the USA and EU ............................................... 6 Controversial Regulatory Concepts: ......................................................................................................... 7 Extrapolation: ........................................................................................................................................ 7 Switching: .............................................................................................................................................. 8 Interchangeability: ................................................................................................................................ 9 Harmonization: .................................................................................................................................... -

Getting to Lower Prescription Drug Prices the Key Drivers of Costs and What Policymakers Can Do to Address Them
Getting to Lower Prescription Drug Prices The Key Drivers of Costs and What Policymakers Can Do to Address Them Henry A. Waxman Bill Corr Jeremy Sharp Ruth McDonald Kahaari Kenyatta OCTOBER 2020 OCTOBER 2020 Getting to Lower Prescription Drug Prices: The Key Drivers of Costs and What Policymakers Can Do to Address Them Henry A. Waxman, Bill Corr, Jeremy Sharp, Ruth McDonald, and Kahaari Kenyatta ABSTRACT ISSUE: Unsustainably high prescription drug prices are a concern for patients, employers, states, and the federal government. There is widespread public support for addressing the problem, and enacting policies to lower drug prices has been a top concern for Congress and the administration over the past three years. Despite this attention, structural changes have not been enacted to rein in drug prices. GOAL: To document the drivers of high U.S. prescription drug prices and offer a broad range of feasible federal policy actions. METHODS: Interviews with experts and organizations engaged on policies related to prescription drug pricing. Review of policy documents, white papers, journal articles, proposals, and position statements. KEY FINDINGS: Action in five areas is key to increasing access to and affordability of medications for Americans: 1) allow the federal government to become a more responsible purchaser; 2) stop patent abuses and anticompetitive practices that block price competition; 3) build a sustainable biosimilar market to create price competition; 4) fix incentives in the drug supply chain and make the supply chain more transparent; and 5) ensure public accountability in the government-funded drug development process. Congress and regulators have a wide range of tools at their disposal to address high drug prices and spending. -

The Process Defines the Product: What Really Matters in Biosimilar Design and Production?
RHEUMATOLOGY Rheumatology 2017;56:iv14iv29 doi:10.1093/rheumatology/kex278 The process defines the product: what really matters in biosimilar design and production? Arnold G. Vulto1 and Orlando A. Jaquez2 Abstract Biologic drugs are highly complex molecules produced by living cells through a multistep manufacturing process. The key characteristics of these molecules, known as critical quality attributes (CQAs), can vary based on post-translational modifications that occur in the cellular environment or during the manufacturing process. The extent of the variation in each of the CQAs must be characterized for the originator molecule and systematically matched as closely as possible by the biosimilar developer to ensure bio-similarity. The close matching of the originator fingerprint is the foundation of the biosimilarity exercise, as the analytical tools designed to measure differences at the molecular level are far more sensitive and specific than tools available to physicians during clinical trials. Biosimilar development, therefore, has a greater focus on preclinical attri- butes compared with the development of an original biological agent. As changes in CQAs can occur at different stages of the manufacturing process, even small modifications to the process can alter biosimilar attributes beyond the point of similarity and impact clinical effectiveness and safety. The manufacturer’s ability to provide consistent production and quality control will greatly influence the acceptance of biosimilars. To this end, preventing drift from the required specifications over time and avoiding the various implications brought by product shortage will enhance biosimilar integration into daily practice. As most prescribers are not familiar with this new drug development paradigm, educational programmes will be needed so that prescribers see biosimilars as fully equivalent, efficacious and safe medicines when compared with originator products. -

Comparison Between Filgrastim and Lenograstim Plus Chemotherapy For
Bone Marrow Transplantation (2010) 45, 277–281 & 2010 Macmillan Publishers Limited All rights reserved 0268-3369/10 $32.00 www.nature.com/bmt ORIGINAL ARTICLE Comparison between filgrastim and lenograstim plus chemotherapy for mobilization of PBPCs R Ria, T Gasparre, G Mangialardi, A Bruno, G Iodice, A Vacca and F Dammacco Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, University of Bari Medical School, Bari, Italy Recombinant human (rHu) G-CSF has been widely used during transplantation.4 However, the use of G-CSF after to treat neutropenia and mobilize PBPCs for their autologous PBPC transplantation has been queried, as its autologous and allogeneic transplantation. It shortens further reduction in time to a safe neutrophil count5,6 does neutropenia and thus reduces the frequency of neutropenic not always imply fewer significant clinical events, such as fever. We compared the efficiency of glycosylated rHu infections, length of hospitalization, extrahematological and non-glycosylated Hu G-CSF in mobilizing hemato- toxicities or mortality.7,8 Even so, the ASCO guidelines still poietic progenitor cells (HPCs). In total, 86 patients were recommend the use of growth factors after autologous consecutively enrolled for mobilization with CY plus either PBPC transplantation.9 glycosylated or non-glycosylated G-CSF, and under- G-CSF induces the proliferation and differentiation of went leukapheresis. The HPC content of each collection, myeloid precursor cells, and also provides a functional toxicity, days of leukapheresis needed to reach the activity that influences chemotaxis, respiratory burst and minimum HPC target and days to recover WBC (X500 Ag expression of neutrophils. -

Filgrastim (Neupogen®); Filgrastim-Aafi (Nivestym™); Filgrastim- Sndz (Zarxio™); Tbo-Filgrastim (Granix®) (Subcutaneous/Intravenous)
Colony Stimulating Factors: Filgrastim (Neupogen®); Filgrastim-aafi (Nivestym™); Filgrastim- sndz (Zarxio™); Tbo-Filgrastim (Granix®) (Subcutaneous/Intravenous) Document Number: DMBA-0235 Last Review Date: 04/01/2020 Date of Origin: 10/17/2008 Dates Reviewed: 06/2009, 12/2009, 06/2010, 07/2010, 09/2010, 12/2010, 03/2011, 6/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016, 02/2017, 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 04/2019, 04/2020 I. Length of Authorization Coverage will be provided for four months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: − Neupogen 300 mcg vial: 3 vials per 1 day − Neupogen 300 mcg SingleJect: 3 syringes per 1 day − Neupogen 480 mcg vial: 3 vials per 1 day − Neupogen 480 mcg SingleJect: 3 syringes per 1 day − Nivestym 300 mcg vial: 3 vials per 1 day − Nivestym 300 mcg prefilled syringe: 3 syringes per 1 day − Nivestym 480 mcg vial: 3 vials per 1 day − Nivestym 480 mcg prefilled syringe: 3 syringes per 1 day − Zarxio 300 mcg prefilled syringe: 3 syringes per 1 day − Zarxio 480 mcg prefilled syringe: 3 syringes per 1 day − Granix 300 mcg pre-filled syringe: 4 syringes per 1 day − Granix 300 mcg single-dose vial: 4 vials per 1 day − Granix 480 mcg pre-filled syringe: 3 syringes per 1 day − Granix 480 mcg single-dose vial: 3 vials per 1 day B. -

G-CSF Protects Motoneurons Against Axotomy-Induced Apoptotic Death In
Henriques et al. BMC Neuroscience 2010, 11:25 http://www.biomedcentral.com/1471-2202/11/25 RESEARCH ARTICLE Open Access G-CSF protects motoneurons against axotomy- induced apoptotic death in neonatal mice Alexandre Henriques1,2,3, Claudia Pitzer1*, Luc Dupuis2,3, Armin Schneider1* Abstract Background: Granulocyte colony stimulating factor (G-CSF) is a growth factor essential for generation of neutrophilic granulocytes. Apart from this hematopoietic function, we have recently uncovered potent neuroprotective and regenerative properties of G-CSF in the central nervous system (CNS). The G-CSF receptor and G-CSF itself are expressed in a motoneurons, G-CSF protects motoneurons, and improves outcome in the SOD1(G93A) transgenic mouse model for amyotrophic lateral sclerosis (ALS). In vitro, G-CSF acts anti- apoptotically on motoneuronal cells. Due to the pleiotrophic effects of G-CSF and the complexity of the SOD1 transgenic ALS models it was however not possible to clearly distinguish between directly mediated anti- apoptotic and indirectly protective effects on motoneurons. Here we studied whether G-CSF is able to protect motoneurons from purely apoptotic cell death induced by a monocausal paradigm, neonatal sciatic nerve axotomy. Results: We performed sciatic nerve axotomy in neonatal mice overexpressing G-CSF in the CNS and found that G-CSF transgenic mice displayed significantly higher numbers of surviving lumbar motoneurons 4 days following axotomy than their littermate controls. Also, surviving motoneurons in G-CSF overexpressing animals were larger, suggesting additional trophic effects of this growth factor. Conclusions: In this model of pure apoptotic cell death the protective effects of G-CSF indicate direct actions of G-CSF on motoneurons in vivo. -

©Ferrata Storti Foundation
Stem Cell Transplantation • Research Paper Single-dose pegfilgrastim for the mobilization of allogeneic CD34+ peripheral blood progenitor cells in healthy family and unrelated donors Frank Kroschinsky Background and Objectives. Short-term treatment with granulocyte colony-stimulating Kristina Hölig factor (G-CSF) has been established as the standard regimen for mobilizing allogeneic Kirsten Poppe-Thiede peripheral blood progenitor cells (PBPC) from healthy donors. The pegylated form of fil- Kristin Zimmer grastim (pegfilgrastim) has a longer elimination half-life because of decreased serum Rainer Ordemann clearance and might be a convenient alternative for stem cell mobilization. Matthias Blechschmidt Uta Oelschlaegel Design and Methods. Twenty-five family (n=15) or unrelated (n=10) healthy donors received a single-dose of 12 mg pegfilgrastim for mobilization of allogeneic PBPC. Martin Bornhauser + Gabi Rall Donors with inadequate mobilization (blood CD34 cells ≤5/µL on day 3 or ≤20/µL on Claudia Rutt day 4) were given additional daily doses of 10 µg/kg conventional filgrastim. Gerhard Ehninger Leukapheresis was planned to start on day 5. Results. All harvests were completed successfully. In 20 out of 25 donors (80 %) only a single apheresis was necessary. Additional non-pegylated filgrastim had to be given to only one 74-year old family donor. The maximum concentration of circulating CD34+ + cells occurred on day 5 (median 67/µL, range 10-385/µL). The median yield of CD34 cells was 9.3 (range 3.2-39.1) 106/kg of the recipient´s body weight. The median × 8 number of T cells in the apheresis products was 3.9 (range 2.7-10.8)×10 /kg. -
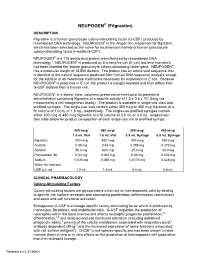
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim Is a Human Granulocyte Colony-Stimulating Factor (G-CSF)‚ Produced by Recombinant DNA Technology
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim is a human granulocyte colony-stimulating factor (G-CSF)‚ produced by recombinant DNA technology. NEUPOGEN® is the Amgen Inc. trademark for filgrastim‚ which has been selected as the name for recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF). NEUPOGEN® is a 175 amino acid protein manufactured by recombinant DNA technology.1 NEUPOGEN® is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NEUPOGEN® has a molecular weight of 18‚800 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NEUPOGEN® is produced in E coli‚ the product is nonglycosylated and thus differs from G-CSF isolated from a human cell. NEUPOGEN is a sterile‚ clear‚ colorless‚ preservative-free liquid for parenteral administration containing filgrastim at a specific activity of 1.0 ± 0.6 x 108 U/mg (as measured by a cell mitogenesis assay). The product is available in single-use vials and prefilled syringes. The single-use vials contain either 300 mcg or 480 mcg filgrastim at a fill volume of 1.0 mL or 1.6 mL, respectively. The single-use prefilled syringes contain either 300 mcg or 480 mcg filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively. See table below for product composition of each single-use vial or prefilled syringe. 300 mcg/ 480 mcg/ 300 mcg/ 480 mcg/ 1.0 mL Vial 1.6 mL Vial 0.5 mL Syringe 0.8 mL Syringe filgrastim 300 mcg 480 mcg 300 mcg 480 mcg Acetate 0.59 mg 0.94 mg 0.295 mg 0.472 mg Sorbitol 50.0 mg 80.0 mg 25.0 mg 40.0 mg Polysorbate 80 0.04 mg 0.064 mg 0.02 mg 0.032 mg Sodium 0.035 mg 0.056 mg 0.0175 mg 0.028 mg Water for Injection USP q.s. -

2019 FDA Biopharmaceutical Approvals: a Record Year for Follow-On Products, but Is Innovation Lagging? Bioplan Associates, Inc
Confidential White Paper – Not for Distribution 2019 FDA Biopharmaceutical Approvals: A Record Year for Follow-on Products, But is Innovation Lagging? BioPlan Associates, Inc. January 2020 Introduction Although 2019 was overall a good year for biopharmaceutical product approvals, there was a lower percentage of approvals for fully new, innovative products compared with prior years. This may indicate problems with recent years’ pharmaceutical industry increased investment in biopharmaceutical vs. drug R&D, with a relative increase in resulting innovative product approvals not showing up yet. It may be too early to make conclusions regarding the extent of this pipeline trend, but the record portion of follow-on product approvals (63% of approvals) can be viewed as positive or negative for the industry. Biopharmaceuticals are here defined as prescription therapeutic products containing active agents manufactured using biotechnology methods, i.e., using living organisms (1). Figure 1 shows the numbers of recombinant and non-recombinant biopharmaceuticals approved by FDA since 1981, when the first recombinant protein received approval. Total biopharmaceutical approvals have increased over the years while remaining steady in the past few years. Figure 1: Numbers of Recombinant and Non-Recombinant Biopharmaceuticals Approved by FDA, 1981-2019 ©2020 BioPlan Associates, Inc, 2275 Research Blvd., Ste. 500, Rockville, MD 20850 (301) 921-5979 Page: 1 Confidential White Paper – Not for Distribution Approvals in 2019 In Table 1 we present approvals during 2019. Several oligonucleotide therapeutics, both antisense and RNAi, also received approval, but these are synthetically manufactured drugs and not considered biopharmaceuticals. Other products not included as biopharmaceuticals include medical devices, allergenic extract products, and diagnostics that receive biologics (BLA) approvals. -

Insulin Glargine-Yfgn Injection) for the Treatment of Diabetes”
Biocon Limited 20th KM, Hosur Road Electronic City Bangalore 560 100, India T 91 80 2808 2808 F 91 80 2852 3423 CIN : L24234KA1978PLC003417 www.biocon.com July 29, 2021 To, To, The Manager The Manager BSE Limited National Stock Exchange of India Limited Department of Corporate Services Corporate Communication Department Phiroze Jeejeebhoy Towers, Exchange Plaza, Bandra Kurla Complex Dalal Street, Mumbai – 400 001 Mumbai – 400 050 Scrip Code – 532523 Scrip Symbol - Biocon Subject: Press Release titled “Biocon Biologics and Viatris Inc. Receive Historic Approval for First Interchangeable Biosimilar Semglee® (insulin glargine-yfgn injection) for the Treatment of Diabetes”. Dear Sir/Madam, Pursuant to Regulation 30 of the SEBI (Listing Obligation and Disclosure Requirements) Regulations, 2015, please find enclosed the press release titled “Biocon Biologics and Viatris Inc. Receive Historic Approval for First Interchangeable Biosimilar Semglee® (insulin glargine-yfgn injection) for the Treatment of Diabetes”. The above information will also be available on the website of the Company at www.biocon.com. Kindly take the same on record and acknowledge. Thanking You, Yours faithfully, For Biocon Limited _______________ Mayank Verma Company Secretary and Compliance Officer Enclosed: A. Press Release B. Press Release done by USFDA: FDA Approved First Interchangeable Biosimilar Insulin Product for treatment of Diabetes, also available at https://www.fda.gov/news-events/press- announcements/fda-approves-first-interchangeable-biosimilar-insulin-product-treatment- diabetes Press Release Biocon Biologics and Viatris Inc. Receive Historic Approval for First Interchangeable Biosimilar Semglee® (insulin glargine-yfgn injection) for the Treatment of Diabetes Interchangeable Designation Allows Substitution at the Pharmacy Counter for Lantus® Across the U.S.