Concerns About the Use of Biosimilar Granulocyte Colony-Stimulating Factors for the Mobilization of Stem Cells in Normal Donors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

TEXAS MEDICAID Clinical Edit Prior Authorization Epoetin Alfa (PROCRIT)
TEXAS MEDICAID Clinical Edit Prior Authorization epoetin alfa (PROCRIT) STEP 1: CLEARLY PRINT AND COMPLETE TO EXPEDITE PROCESSING Date: Prescriber First & Last Name: Patient First & Last Name: Prescriber NPI: Patient Address: Prescriber Address: Patient ID: Prescriber Phone: Patient Date of Birth: Prescriber Fax: STEP 2: MEDICATION INFORMATION Medication Requested (Name): Quantity Requested: Dose Requested: Dosing Instructions: Patient’s Primary Diagnosis: ____________________________________ ICD 10 Code: __________ Please indicate ONE (1) of the following: OR STAR / STAR KIDS client (Go to Step 3 - PDL PA Criteria Applies) OR CHIP / PERINATE client (Go to Step 4) STEP 3: PDL PRIOR AUTHORIZATION CRITERIA FOR NON-PREFERRED PRODUCT 1. Has the client failed a 30-day treatment trial with at least 1 preferred agent in the last 180 days? Yes (Go to Step 4 Question 1) No (Go to #2) 2. Is there a documented allergy or contraindication to preferred agents in this class? Yes (Go to Step 4 Question 1) No (Go to #3) 3. Is the drug necessary for treatment of stage-4 advanced metastatic cancer and associated conditions? Yes (Go to Step 4 Question 1) No (Deny) Rev. 11/18/2020 Page 1 of 3 Version 1.5 STEP 4: CLINICAL PRIOR AUTHORIZATION CRITERIA 1. Does the client have a diagnosis of chronic renal failure in the last 730 days? Yes (Go to #7) No (Go to #2) 2. Does the client have a diagnosis of cancer in the last 730 days? Yes (Go to #3) No (Go to #5) 3. Does the client have a history of an antineoplastic agent in the last 30 days? Examples of antineoplastic -

The Evolution of Biosimilars in Oncology, with a Focus on Trastuzumab
EVOLUTIONPERSPECTIVES OF BIOSIMILARS IN INONCOLOGY, ONCOLOGY Nixon et al. The evolution of biosimilars in oncology, with a focus on trastuzumab †‡ N.A. Nixon MD,* M.B. Hannouf PhD, and S. Verma MD* ABSTRACT Cancer therapy has evolved significantly with increased adoption of biologic agents (“biologics”). That evolution is especially true for her2 (human epidermal growth factor receptor-2)–positive breast cancer with the introduction of trastuzumab, a monoclonal antibody against the her2 receptor, which, in combination with chemotherapy, significantly improves survival in both metastatic and early disease. Although the efficacy of biologics is undeniable, their expense is a significant contributor to the increasing cost of cancer care. Across disease sites and indications, biosimilar agents are rapidly being developed with the goal of offering cost-effective alternatives to biologics. Biosimilars are pharmaceuticals whose molecular shape, efficacy, and safety are similar, but not identical, to those of the original product. Although these agents hold the potential to improve patient access, complexities in their production, evaluation, cost, and clinical application have raised questions among experts. Here, we review the landscape of biosimilar agents in oncology, with a focus on trastu- zumab biosimilars. We discuss important considerations that must be made as these agents are introduced into routine cancer care. Key Words Biosimilars, trastuzumab, Herceptin, value Curr Oncol. 2018 Jun;25(S1):S171-S179 www.current-oncology.com INTRODUCTION alternatives to biologics. Although these agents have the potential to improve patient access, complexities in their In recent years, cancer treatment has been revolutionized production, evaluation, and clinical application have raised by the introduction of biologic agents (“biologics”). -

Biopharmaceutical Notes
An Overview of the BioPharmaceutical Products and Market By Paul DiMarco, Vice President, Global Commercial Program, BioSpectra Inc. Table of Contents INTRODUCTION: ........................................................................................................................................ 2 History ....................................................................................................................................................... 2 Background information ........................................................................................................................... 3 Defining biological products ..................................................................................................................... 4 Small vs. Large Molecule Regulation and Registration in the USA and EU ............................................... 6 Controversial Regulatory Concepts: ......................................................................................................... 7 Extrapolation: ........................................................................................................................................ 7 Switching: .............................................................................................................................................. 8 Interchangeability: ................................................................................................................................ 9 Harmonization: .................................................................................................................................... -

Getting to Lower Prescription Drug Prices the Key Drivers of Costs and What Policymakers Can Do to Address Them
Getting to Lower Prescription Drug Prices The Key Drivers of Costs and What Policymakers Can Do to Address Them Henry A. Waxman Bill Corr Jeremy Sharp Ruth McDonald Kahaari Kenyatta OCTOBER 2020 OCTOBER 2020 Getting to Lower Prescription Drug Prices: The Key Drivers of Costs and What Policymakers Can Do to Address Them Henry A. Waxman, Bill Corr, Jeremy Sharp, Ruth McDonald, and Kahaari Kenyatta ABSTRACT ISSUE: Unsustainably high prescription drug prices are a concern for patients, employers, states, and the federal government. There is widespread public support for addressing the problem, and enacting policies to lower drug prices has been a top concern for Congress and the administration over the past three years. Despite this attention, structural changes have not been enacted to rein in drug prices. GOAL: To document the drivers of high U.S. prescription drug prices and offer a broad range of feasible federal policy actions. METHODS: Interviews with experts and organizations engaged on policies related to prescription drug pricing. Review of policy documents, white papers, journal articles, proposals, and position statements. KEY FINDINGS: Action in five areas is key to increasing access to and affordability of medications for Americans: 1) allow the federal government to become a more responsible purchaser; 2) stop patent abuses and anticompetitive practices that block price competition; 3) build a sustainable biosimilar market to create price competition; 4) fix incentives in the drug supply chain and make the supply chain more transparent; and 5) ensure public accountability in the government-funded drug development process. Congress and regulators have a wide range of tools at their disposal to address high drug prices and spending. -

The Process Defines the Product: What Really Matters in Biosimilar Design and Production?
RHEUMATOLOGY Rheumatology 2017;56:iv14iv29 doi:10.1093/rheumatology/kex278 The process defines the product: what really matters in biosimilar design and production? Arnold G. Vulto1 and Orlando A. Jaquez2 Abstract Biologic drugs are highly complex molecules produced by living cells through a multistep manufacturing process. The key characteristics of these molecules, known as critical quality attributes (CQAs), can vary based on post-translational modifications that occur in the cellular environment or during the manufacturing process. The extent of the variation in each of the CQAs must be characterized for the originator molecule and systematically matched as closely as possible by the biosimilar developer to ensure bio-similarity. The close matching of the originator fingerprint is the foundation of the biosimilarity exercise, as the analytical tools designed to measure differences at the molecular level are far more sensitive and specific than tools available to physicians during clinical trials. Biosimilar development, therefore, has a greater focus on preclinical attri- butes compared with the development of an original biological agent. As changes in CQAs can occur at different stages of the manufacturing process, even small modifications to the process can alter biosimilar attributes beyond the point of similarity and impact clinical effectiveness and safety. The manufacturer’s ability to provide consistent production and quality control will greatly influence the acceptance of biosimilars. To this end, preventing drift from the required specifications over time and avoiding the various implications brought by product shortage will enhance biosimilar integration into daily practice. As most prescribers are not familiar with this new drug development paradigm, educational programmes will be needed so that prescribers see biosimilars as fully equivalent, efficacious and safe medicines when compared with originator products. -

UWHC Guidelines for the Use of Darbepoetin and Epoetin
Use of Darbepoetin and Epoetin in Non-Nephrology Patients – Adult/Pediatric – Inpatient/Ambulatory Clinical Practice Guideline Table of Contents Executive Summary ......................................................................................... 3 Scope ............................................................................................................. 7 Methodology ................................................................................................... 7 Definitions (optional): ...................................................................................... 8 Introduction.................................................................................................... 8 Recommendations........................................................................................... 9 UW Health Implementation............................................................................ 19 References ................................................................................................... 19 Note: Active Table of Contents Click to follow link CPG Contact for Changes: Name: Philip Trapskin, PharmD, BCPS, Manager, DPP Phone Number: 608-263-1328 Email address: [email protected] CPG Contact for Content: Name: Jason Bergsbaken, PharmD Phone Number: 608-265-0341 Email address: [email protected] Copyright © 2015 University of Wisconsin Hospitals and Clinics Authority Contact: [email protected] Vermeulen, [email protected] Last Revised: 07/2015 Guideline Authors: Jason Bergsbaken, PharmD Coordinating -

Filgrastim (Neupogen®); Filgrastim-Aafi (Nivestym™); Filgrastim- Sndz (Zarxio™); Tbo-Filgrastim (Granix®) (Subcutaneous/Intravenous)
Colony Stimulating Factors: Filgrastim (Neupogen®); Filgrastim-aafi (Nivestym™); Filgrastim- sndz (Zarxio™); Tbo-Filgrastim (Granix®) (Subcutaneous/Intravenous) Document Number: DMBA-0235 Last Review Date: 04/01/2020 Date of Origin: 10/17/2008 Dates Reviewed: 06/2009, 12/2009, 06/2010, 07/2010, 09/2010, 12/2010, 03/2011, 6/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016, 02/2017, 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 04/2019, 04/2020 I. Length of Authorization Coverage will be provided for four months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: − Neupogen 300 mcg vial: 3 vials per 1 day − Neupogen 300 mcg SingleJect: 3 syringes per 1 day − Neupogen 480 mcg vial: 3 vials per 1 day − Neupogen 480 mcg SingleJect: 3 syringes per 1 day − Nivestym 300 mcg vial: 3 vials per 1 day − Nivestym 300 mcg prefilled syringe: 3 syringes per 1 day − Nivestym 480 mcg vial: 3 vials per 1 day − Nivestym 480 mcg prefilled syringe: 3 syringes per 1 day − Zarxio 300 mcg prefilled syringe: 3 syringes per 1 day − Zarxio 480 mcg prefilled syringe: 3 syringes per 1 day − Granix 300 mcg pre-filled syringe: 4 syringes per 1 day − Granix 300 mcg single-dose vial: 4 vials per 1 day − Granix 480 mcg pre-filled syringe: 3 syringes per 1 day − Granix 480 mcg single-dose vial: 3 vials per 1 day B. -

Dr. Winegarden Presentation
Empowering Market Competition through Biosimilars Presentation to National Council of Insurance Legislators (NCOIL) 2019 Summer Meeting Newport Beach, California July 12, 2019 Total Savings (in millions) 25% 50% 75% Originator Total Annual Savings for Current, 25%, Drug Class Current Biosimilar Biosimilar Biosimilar Biologic 50%, and 75% Biosimilar Share Share Share Share Scenarios Compared to All-Originator Infliximab Remicade $79.4 $318.2 $636.5 $954.7 Biologic Baseline Pegfilgrastim Neulasta $21.8 $121.9 $243.8 $365.7 Filgrastim Neupogen $152.1 $152.1 $152.1 $206.8 Epoetin Alfa Epogen & Procrit $0.5 $8.4 $16.9 $25.3 Bevacizumab Avastin $0.0 $199.2 $398.5 $597.7 Trastuzumab Herceptin $0.0 $208.0 $415.9 $623.9 • Biosimilars price discounts expected 30% - Rituxumab Rituxan $0.0 $280.6 $561.2 $841.8 40% off originator biologic Etanercept Enbrel $0.0 $324.0 $648.0 $972.1 • Forthcoming study estimates possible Adalimunab Humira $0.0 $861.1 $1,722.1 $2,583.2 health care savings from wider adoption of biosimilars. GRAND TOTAL $253.8 $2,473.6 $4,795.0 $7,171.2 • Estimates are based on the average sales price (ASP) data that are effective from April 2019 through June 2019 and rolling 12-month volume data through February 2019. Biosimilars have no “clinically meaningful difference” in safety, purity, • Methodology: compare potential savings to hypothetical all-originator biologic scenario and effectiveness relative to its reference originator biologic. Just like • Over 10 years, potential savings of $24.7 generic medicines, the benefits of biosimilars are the substantial price billion, $48.0 billion, and $71.7 billion respectively. -
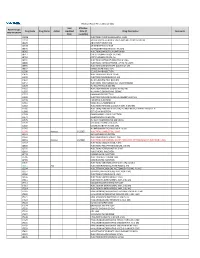
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

2019 FDA Biopharmaceutical Approvals: a Record Year for Follow-On Products, but Is Innovation Lagging? Bioplan Associates, Inc
Confidential White Paper – Not for Distribution 2019 FDA Biopharmaceutical Approvals: A Record Year for Follow-on Products, But is Innovation Lagging? BioPlan Associates, Inc. January 2020 Introduction Although 2019 was overall a good year for biopharmaceutical product approvals, there was a lower percentage of approvals for fully new, innovative products compared with prior years. This may indicate problems with recent years’ pharmaceutical industry increased investment in biopharmaceutical vs. drug R&D, with a relative increase in resulting innovative product approvals not showing up yet. It may be too early to make conclusions regarding the extent of this pipeline trend, but the record portion of follow-on product approvals (63% of approvals) can be viewed as positive or negative for the industry. Biopharmaceuticals are here defined as prescription therapeutic products containing active agents manufactured using biotechnology methods, i.e., using living organisms (1). Figure 1 shows the numbers of recombinant and non-recombinant biopharmaceuticals approved by FDA since 1981, when the first recombinant protein received approval. Total biopharmaceutical approvals have increased over the years while remaining steady in the past few years. Figure 1: Numbers of Recombinant and Non-Recombinant Biopharmaceuticals Approved by FDA, 1981-2019 ©2020 BioPlan Associates, Inc, 2275 Research Blvd., Ste. 500, Rockville, MD 20850 (301) 921-5979 Page: 1 Confidential White Paper – Not for Distribution Approvals in 2019 In Table 1 we present approvals during 2019. Several oligonucleotide therapeutics, both antisense and RNAi, also received approval, but these are synthetically manufactured drugs and not considered biopharmaceuticals. Other products not included as biopharmaceuticals include medical devices, allergenic extract products, and diagnostics that receive biologics (BLA) approvals.