Ecology of Black Bears on the Beartooth Face, South-Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pines in the Arboretum
UNIVERSITY OF MINNESOTA MtJ ARBORETUM REVIEW No. 32-198 PETER C. MOE Pines in the Arboretum Pines are probably the best known of the conifers native to The genus Pinus is divided into hard and soft pines based on the northern hemisphere. They occur naturally from the up the hardness of wood, fundamental leaf anatomy, and other lands in the tropics to the limits of tree growth near the Arctic characteristics. The soft or white pines usually have needles in Circle and are widely grown throughout the world for timber clusters of five with one vascular bundle visible in cross sec and as ornamentals. In Minnesota we are limited by our cli tions. Most hard pines have needles in clusters of two or three mate to the more cold hardy species. This review will be with two vascular bundles visible in cross sections. For the limited to these hardy species, their cultivars, and a few hy discussion here, however, this natural division will be ignored brids that are being evaluated at the Arboretum. and an alphabetical listing of species will be used. Where neces Pines are readily distinguished from other common conifers sary for clarity, reference will be made to the proper groups by their needle-like leaves borne in clusters of two to five, of particular species. spirally arranged on the stem. Spruce (Picea) and fir (Abies), Of the more than 90 species of pine, the following 31 are or for example, bear single leaves spirally arranged. Larch (Larix) have been grown at the Arboretum. It should be noted that and true cedar (Cedrus) bear their leaves in a dense cluster of many of the following comments and recommendations are indefinite number, whereas juniper (Juniperus) and arborvitae based primarily on observations made at the University of (Thuja) and their related genera usually bear scalelikie or nee Minnesota Landscape Arboretum, and plant performance dlelike leaves that are opposite or borne in groups of three. -

Douglas- Fir Limber Pine Lodgepole Pine Ponderosa Pine Blue Spruce
NAME ORIGIN BARK FEMALE CONES NEEDLES WHERE USES TRIVIA Named by Smooth gray bark To 4ÂÂ long, Soft, flat, 2-sided, Found on north or Railroad crossties, State tree of Scottish botanist on young trees yellowish to light 1¼″ long and south-facing slopes, mine timbers, for Oregon. David Douglas. with numerous brown hanging cones rounded at the tip. in shady ravines and building ships and The Latin name DOUGLAS- Fir is from the resin scars. with uniquely 3- Dark yellow green or on rocky slopes boats, construction psuedotsuga Middle English pointed bracts blue green. Shortly where the soil is lumber, plywood, means FIR firre and Old protruding from cone stalked spreading fairly deep. telephone poles, ÂÂfalse Psuedotsuga English fyrh. scales like a snakes- mostly in two rows. fencing, railroad-car fir.ÂÂ menziesii tongue. Single small groove construction, boxes Can drop 2 on topside of needles and crates, flooring, million seeds in and single white line furniture, ladders a good year. on underside of and pulpwood. needles. Pine is from the Light gray to Big (to 9ÂÂ long) Stout in clusters of 5 Found on rocky, Lumber, railroad Cones start to LIMBER Latin pinus and blackish brown. cylindrical, greenish needles, to 3″ long. gravelly slopes, cross ties, poles, appear after the the Old English Smooth and silvery brown, with thick, Straight or slightly ridges and peaks. turpentine, tar and tree reaches 20 PINE pin. gray on young broad scales. Cone curved, not sticky to fuel. years of age. Pinus flexilis trees. scales lack prickles. the touch. Dark green. Pine is from the Bark is grayish or Light yellow brown, Stout, twisted Found in well Lumber, knotty Many Plains Latin pinus and light brown, thin reddish or dark green, needles, mostly in drained soils, dry pine paneling, Indian tribes the Old English and with many lopsided cones to pairs, to 2½″ long. -

Quaternary Research 79 (2013) 309
Quaternary Research 79 (2013) 309 Contents lists available at SciVerse ScienceDirect Quaternary Research journal homepage: www.elsevier.com/locate/yqres Corrigendum Corrigendum to “Late-Holocene response of limber pine (Pinus flexilis) forests to fire disturbance in the Pine Forest Range, Nevada, USA” [Quaternary Research 78 (2012) 465–473] Robert K. Shriver a,1, Thomas A. Minckley a,b,⁎ a Dept. of Botany, University of Wyoming, Laramie, WY 82071, USA b Roy J. Shlemon Center for Quaternary Studies, University of Wyoming, Laramie, WY 82071, USA The purpose of Shriver and Minckley (2012) “Late-Holocene re- The occurrence of such a large population of whitebark pine in this sponse of limber pine (Pinus flexilis) forests to fire disturbance in portion of northwestern Nevada is notable in itself. The Pine Forest the Pine Forest Range, Nevada, USA” was to assess historic responses Range is not particularly high in comparison to surrounding ranges to disturbance (fire) using pollen percentage data derived from a that do not have similar forest types. The presence of a few but exceed- sediment core using superimposed epoch analysis. This work was ingly rare limber pines suggests that there might have been historic conducted in a small glacial tarn, Blue Lake, located in an isolated processes that have favored one species versus the other over time — mountain range of northwestern Nevada, the Pine Forest Range. maybe even fire. The differences in the ecology of the two species are The benefit of this site was the unique setting of an isolated forest significant enough to suspect that they would have different climatic that added to our knowledge of disturbance in five-needle pine eco- and disturbance responses. -

Conservation Genetics of High Elevation Five-Needle White Pines
Conservation Genetics of High Elevation Five-Needle White Pines Conservation Genetics of High Elevation Five-Needle White Pines Andrew D. Bower, USDA Forest Service, Olympic National Forest, Olympia, WA; Sierra C. McLane, University of British Columbia, Dept. of Forest Sciences, Vancouver, BC; Andrew Eckert, University of California Davis, Section of Plenary Paper Evolution and Ecology, Davis, CA; Stacy Jorgensen, University of Hawaii at Manoa, Department of Geography, Manoa, HI; Anna Schoettle, USDA Forest Service, Rocky Mountain Research Station, Fort Collins, CO; Sally Aitken, University of British Columbia, Dept. of Forest Sciences, Vancouver, BC Abstract—Conservation genetics examines the biophysical factors population structure using molecular markers and quanti- influencing genetic processes and uses that information to conserve tative traits and assessing how these measures are affected and maintain the evolutionary potential of species and popula- by ecological changes. Genetic diversity is influenced by the tions. Here we review published and unpublished literature on the evolutionary forces of mutation, selection, migration, and conservation genetics of seven North American high-elevation drift, which impact within- and among-population genetic five-needle pines. Although these species are widely distributed across much of western North America, many face considerable diversity in differing ways. Discussions of how these forces conservation challenges: they are not valued for timber, yet they impact genetic diversity can be found in many genetics texts have high ecological value; they are susceptible to the introduced (for example Frankham and others 2002; Hartl and Clark disease white pine blister rust (caused by the fungus Cronartium 1989) and will not be discussed here. ribicola) and endemic-turned-epidemic pests; and some are affect- ed by habitat fragmentation and successional replacement by other Why Is Genetic Diversity Important? species. -

Distribution of the Native Trees of Utah Kimball S
Brigham Young University Science Bulletin, Biological Series Volume 11 | Number 3 Article 1 9-1970 Distribution of the native trees of Utah Kimball S. Erdman Department of Biology, Slippery Rock State College, Slippery Rock, Pennsylvania Follow this and additional works at: https://scholarsarchive.byu.edu/byuscib Part of the Anatomy Commons, Botany Commons, Physiology Commons, and the Zoology Commons Recommended Citation Erdman, Kimball S. (1970) "Distribution of the native trees of Utah," Brigham Young University Science Bulletin, Biological Series: Vol. 11 : No. 3 , Article 1. Available at: https://scholarsarchive.byu.edu/byuscib/vol11/iss3/1 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Brigham Young University Science Bulletin, Biological Series by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. MU3. CCy.P. ZOOL. LIBRARY DEC 41970 Brigham Young University HARVARD Science Bulletin UNIVERSITY) DISTRIBUTION OF THE NATIVE TREES OF UTAH by Kimball S. Erdman BIOLOGICAL SERIES—VOLUME XI, NUMBER 3 SEPTEMBER 1970 BRIGHAM YOUNG UNIVERSITY SCIENCE BULLETIN BIOLOGICAL SERIES Editor: Stanley L. Welsh, Department of Botany, Brigham Young University, Provo, Utah Members of the Editorial Board: Tipton, Zoology Vernon J. Feeeon L. Anderson, Zoology Joseph R. Murdock, Botany WiLMER W. Tanner, Zoology Ex officio Members: A. Lester Allen, Dean, College of Biological and Agricultural Sciences Ernest L. Olson, Chairman, University Publications The Brigham Young University Science Bulletin, Biological Series, publishes acceptable papers, particularly large manuscripts, on all phases of biology. Separate numbers and back volumes can be purchased from Pubhcation Sales, Brigham Young University, Provo, Utah. -

Status and Dynamics of Whitebark Pine (Pinus Albicaulis Engelm.) Forests in Southwest Montana, Central Idaho, and Oregon, U.S.A
Status and Dynamics of Whitebark Pine (Pinus albicaulis Engelm.) Forests in Southwest Montana, Central Idaho, and Oregon, U.S.A. A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Evan Reed Larson IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Kurt F. Kipfmueller June 2009 © Evan Reed Larson 2009 Acknowledgements This research was made possible through the efforts of a number of people. For their help in the field, my thanks go to Kyle Anderson, Adam Berland, Brad Bogard, Neil Green-Clancey, Noelle Harden, Zack and Mesa Holmboe, Matt Jacobson, Eric and Shelley Larson, Tony and Donna Praza, Danica and Mara Larson, Karen Arabas, Joe Bowersox, and the Forest Ecology class from Willamette University including Eric Autrey, Luke Barron, Jeff Bennett, Laura Cattrall, Maureen Goltz, Whitney Pryce, Maria Savoca, Hannah Wells, and Kaitlyn Wright. Thanks to Jessica Burke, Noelle Harden, and Jens Loberg for their long hours helping sand my samples to a high shine. Many thanks also to USDA Forest Service personnel Carol Aubrey, Kristen Chadwick, Vickey Erickson, Carly Gibson, Bill Given, Robert Gump, Chris Jensen, Bob Keane, Al Kyles, Clark Lucas, Robin Shoal, David Swanson, Sweyn Wall, and Bob Wooley for their time and assistance in planning logistics and gaining sampling permission for my research. I am fortunate to have been influenced by many wonderful friends and mentors during my academic career. I give thanks to my Ph.D. committee members Lee Frelich, Kathy Klink, Bryan Shuman, and Susy Ziegler for their guidance and efforts on my behalf. -

Status of the Limber Pine (Pinus Flexilis)
Status of the Limber Pine (3LQXVÀH[LOLV) in Alberta Alberta Wildlife Status Report No. 62 Status of the Limber Pine (3LQXVÀH[LOLV) in Alberta Prepared for: Alberta Sustainable Resource Development (SRD) Alberta Conservation Association (ACA) Prepared by: David W. Langor This report has been reviewed, revised, and edited prior to publication. It is an SRD/ACA working document that will be revised and updated periodically. Alberta Wildlife Status Report No. 62 June 2007 Published By: i Publication No. T/140 ISBN: 978-0-7785-6487-4 (Printed Edition) ISBN: 978-0-7785-6488-1 (On-line Edition) ISSN: 1206-4912 (Printed Edition) ISSN: 1499-4682 (On-line Edition) Series Editors: Sue Peters, Robin Gutsell, Nyree Sharp and Lisa Matthias Illustrations: Brian Huffman Maps: Nicole Hopkins For copies of this report, visit our web site at: KWWSVUGDOEHUWDFD¿VKZLOGOLIHVSHFLHVDWULVN and click on “Detailed Status” OR Contact: Information Centre - Publications Alberta Sustainable Resource Development Main Floor, Great West Life Building 9920 - 108 Street Edmonton, Alberta, Canada T5K 2M4 Telephone: (780) 422-2079 This publication may be cited as: Alberta Sustainable Resource Development and Alberta Conservation Association. 2007. Status of the limber pine (3LQXVÀH[LOLV) in Alberta. Alberta Sustainable Resource Development, Wildlife Status Report No. 62, Edmonton, AB. 17 pp. ii PREFACE (YHU\¿YH\HDUVWKH)LVKDQG:LOGOLIH'LYLVLRQRI$OEHUWD6XVWDLQDEOH5HVRXUFH'HYHORSPHQW reviews the general status of wildlife species in Alberta. These overviews, which have been conducted in 1991 (The Status of Alberta Wildlife), 1996 (The Status of Alberta Wildlife), 2000 (The General Status of Alberta Wild Species 2000), and 2005 (The General Status of Alberta Wild Species 2005 DVVLJQLQGLYLGXDOVSHFLHV³UDQNV´WKDWUHÀHFWWKHSHUFHLYHGOHYHORIULVNWRSRSXODWLRQV that occur in the province. -

First Report of Two Cone and Seed Insects on Pinus Flexilis
Western North American Naturalist Volume 61 Number 2 Article 15 4-23-2001 First report of two cone and seed insects on Pinus flexilis A. W. Schoettle Rocky Mountain Research Station, Fort Collins, Colorado J. F. Negron Rocky Mountain Research Station, Fort Collins, Colorado Follow this and additional works at: https://scholarsarchive.byu.edu/wnan Recommended Citation Schoettle, A. W. and Negron, J. F. (2001) "First report of two cone and seed insects on Pinus flexilis," Western North American Naturalist: Vol. 61 : No. 2 , Article 15. Available at: https://scholarsarchive.byu.edu/wnan/vol61/iss2/15 This Note is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Western North American Naturalist 61(2), © 2001, pp. 252–254 FIRST REPORT OF TWO CONE AND SEED INSECTS ON PINUS FLEXILIS A.W. Schoettle1 and J.F. Negron1 Key words: Coleoptera, cone and seed insects, Conophthorus contortae, Dioryctria auranticella, Hemiptera, Lepi- doptera, Leptoglossus occidentalis, limber pine, Pinus flexilis. Limber pine (Pinus flexilis James) ranges in D. auranticella in ponderosa pine in north latitude from 33°N to 51°N and in elevation central Colorado. Dioryctria auranticella has from 870 m above sea level (asl) in North previously been reported on knobcone (Pinus Dakota to ~3400 m asl in Colorado (Burns attenuata Lemm.), ponderosa (Pinus ponderosa and Honkala 1990). In the central Rocky Dougl. ex Laws.), radiata (Pinus radiata D. -

Sustaining High Elevation Five-Needle Pines in the Southwestern United States: a Practical Management Perspective
Sustaining High Elevation Five-Needle Pines in the Southwestern United States: A Practical Management Perspective Tara Steadman A PROFESSIONAL PAPER SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF FORESTRY Northern Arizona University May 2018 Approved: Kristen Waring, Ph.D., Major Advisor Thomas Kolb, Ph.D. Arthur Haines, Certified Silviculturist 1 Contents Abstract .............................................................................................................................................3 Introduction ......................................................................................................................................4 Silvics and Associated Cover Types .....................................................................................................6 Southwestern white pine ................................................................................................................6 Limber pine ....................................................................................................................................8 Rocky Mountain bristlecone ............................................................................................................9 Great Basin bristlecone ................................................................................................................. 10 Fire Ecology and Regeneration ................................................................................................... 11 Primary Threats .............................................................................................................................. -
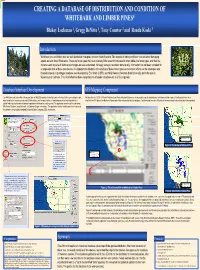
CREATING a DATABASE of DISTRIBUTION and CONDITION of WHITEBARK and LIMBER PINES4 Blakey Lockman 1, Gregg Denitto 1, Tony Courter 2 and Ronda Koski 3
CREATING A DATABASE OF DISTRIBUTION AND CONDITION OF WHITEBARK AND LIMBER PINES4 Blakey Lockman 1, Gregg DeNitto 1, Tony Courter 2 and Ronda Koski 3 Introduction Whitebark pine and limber pine are well distributed throughout western North America. The impacts of white pine blister rust and other damaging agents are also found West-wide. These are forest types that have relatively little research compared to other white pine forest types, and thus the dynamics and impacts of detrimental changes are less understood. Although surveys have been done locally, information has not been compiled for a range-wide look at these two species. It is important to determine the condition of these forest types so restoration efforts can be developed and focused properly. A prototype database was developed by Eric Smith (USFS) and Holly Kearns (Colorado State University) for limber pine in Marcus Jackson Wyoming and Colorado. This initial effort has been expanded into a broader database with a GIS component. Maria Newcomb Database/Interface Development GIS/Mapping Component The Whitebark and Limber Pine Information System (WBLIS) consists of a limited number of critical fields representing key plot Plot data from the USFS FIA Pacific Northwest and Rocky Mountain Stations are being used to map the distribution of whitebark and limber pines. Condition data have been data variables that can be queried and GIS-linked via a user-friendly interface. A workshop was held to develop this list of extracted from FIA plots in the Northern Region and will be incorporated into the database. Condition data from other FIA plots in the west remain to be extracted and incorporated. -

Pinus Flexilis) to Multiyear Droughts and 20Th-Century Warming, Sierra Nevada, California, USA
2508 Response of high-elevation limber pine (Pinus flexilis) to multiyear droughts and 20th-century warming, Sierra Nevada, California, USA Constance I. Millar, Robert D. Westfall, and Diane L. Delany Abstract: Limber pine (Pinus flexilis James) stands along the eastern escarpment of the Sierra Nevada, California, experi- enced significant mortality from 1985 to 1995 during a period of sustained low precipitation and high temperature. The stands differ from old-growth limber pine forests in being dense, young, more even-aged, and located in warmer, drier mi- croclimates. Tree growth showed high interannual variability. Relative to live trees, dead trees over their lifetimes had higher series sensitivity, grew more variably, and had lower growth. Although droughts recurred during the 20th century, tree mortality occurred only in the late 1980s. Significant correlations and interactions of growth and mortality dates with temperature and precipitation indicate that conditions of warmth plus sustained drought increased the likelihood of mortal- ity in the 1985–1995 interval. This resembles a global-change-type drought, where warming combined with drought was an initial stress, trees were further weakened by dwarf mistletoe (Arceuthobium cyanocarpum (A. Nels. ex Rydb.) A. Nels.), and proximally killed by mountain pine beetle (Dendroctonus ponderosae Hopkins). However, the thinning effect of the drought-related mortality appears to have promoted resilience and improved near-term health of these stands, which suffered no additional mortality in the subsequent 1999–2004 drought. Re´sume´ : Les peuplements de pin flexible (Pinus flexilis James) situe´s le long de l’escarpement est de la Sierra Nevada, en Californie, ont subi une mortalite´ appre´ciable de 1985 a` 1995 durant une pe´riode marque´e par des conditions soutenues de faible pre´cipitation et de tempe´rature e´leve´e. -

Native and Naturalized Conifers of Nevada a Checklist and Description
COOPERATIVE EXTENSION Bringing the University to You Special Publication 06-05 NATIVE AND NATURALIZED CONIFERS OF NEVADA A CHECKLIST AND DESCRIPTION Wayne S Johnson, Associate Professor, Department of Resource Economics, College of Agriculture, Biotechnology & Natural Resources; Urban Forestry Specialist, University of Nevada Cooperative Extension Sue Strom, Urban Forestry Program Assistant, University of Nevada, Reno Patricia Rowley, Urban Forestry Volunteer Development Coordinator, University of Nevada Cooperative Extension Taylor Nelson, Research Assistant, University of Nevada, Reno This checklist is offered to increase year. With much adaptability among species, awareness of native and naturalized conifers some conifers can survive in very low present in Nevada. Native conifers are those precipitation zones. However, most plantings found growing naturally in Nevada. require supplemental irrigation during and after Naturalized conifers are those introduced into establishment for best tree performance in a Nevada that are reproducing without human landscape. assistance in natural environs. They grow Junipers and the subalpine pines tolerate a above valleys on steppe or mountain slopes, wider range of soil salts than other conifers but many of these trees can be grown in urban found in Nevada. The alpine conifers prefer and community forests throughout Nevada. acid soils, but with few exceptions will grow in This is particularly so when large parks, travel soils with a pH less than pH 8. Most of the rest and utility corridors, and other areas are being tolerate the moderate alkaline soils in Nevada. restored or left “natural.” Native and naturalized plant species, such With few exceptions, when planting native as conifers, are important to all forms of life, and naturalized conifers, the site should mimic including vertebrates (large and small the natural environment of the species as mammals, birds, and fish) and invertebrates closely as possible.