Two New Eriophyid Mite Species Associated with Clematis Terniflora Var
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Spring 5-2016 Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi Hanna M. Miller University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Biodiversity Commons, and the Botany Commons Recommended Citation Miller, Hanna M., "Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi" (2016). Honors Theses. 389. https://aquila.usm.edu/honors_theses/389 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi by Hanna Miller A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirement for the Degree of Bachelor of Science in the Department of Biological Sciences May 2016 ii Approved by _________________________________ Mac H. Alford, Ph.D., Thesis Adviser Professor of Biological Sciences _________________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences _________________________________ Ellen Weinauer, Ph.D., Dean Honors College iii Abstract The North American Coastal Plain contains some of the highest plant diversity in the temperate world. However, most of the region has remained unstudied, resulting in a lack of knowledge about the unique plant communities present there. -
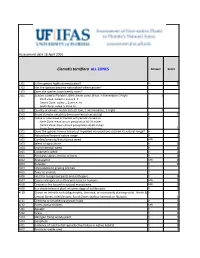
WRA.Datasheet.Template (Version 1) (Version 1).Xlsx
Assessment date 18 April 2016 Clematis terniflora ALL ZONES Answer Score 1.01 Is the species highly domesticated? n 0 1.02 Has the species become naturalised where grown? 1.03 Does the species have weedy races? 2.01 Species suited to Florida's USDA climate zones (0-low; 1-intermediate; 2-high) 2 North Zone: suited to Zones 8, 9 Central Zone: suited to Zones 9, 10 South Zone: suited to Zone 10 2.02 Quality of climate match data (0-low; 1-intermediate; 2-high) 2 2.03 Broad climate suitability (environmental versatility) y 1 2.04 Native or naturalized in habitats with periodic inundation y North Zone: mean annual precipitation 50-70 inches Central Zone: mean annual precipitation 40-60 inches South Zone: mean annual precipitation 40-60 inches 1 2.05 Does the species have a history of repeated introductions outside its natural range? y 3.01 Naturalized beyond native range y 2 3.02 Garden/amenity/disturbance weed unk 3.03 Weed of agriculture n 0 3.04 Environmental weed y 4 3.05 Congeneric weed y 2 4.01 Produces spines, thorns or burrs n 0 4.02 Allelopathic unk 0 4.03 Parasitic n 0 4.04 Unpalatable to grazing animals ? 4.05 Toxic to animals ? 4.06 Host for recognised pests and pathogens n 0 4.07 Causes allergies or is otherwise toxic to humans unk 0 4.08 Creates a fire hazard in natural ecosystems unk 0 4.09 Is a shade tolerant plant at some stage of its life cycle n 0 4.10 Grows on infertile soils (oligotrophic, limerock, or excessively draining soils). -

State of Delaware Invasive Plants Booklet
Planting for a livable Delaware Widespread and Invasive Growth Habit 1. Multiflora rose Rosa multiflora S 2. Oriental bittersweet Celastrus orbiculata V 3. Japanese stilt grass Microstegium vimineum H 4. Japanese knotweed Polygonum cuspidatum H 5. Russian olive Elaeagnus umbellata S 6. Norway maple Acer platanoides T 7. Common reed Phragmites australis H 8. Hydrilla Hydrilla verticillata A 9. Mile-a-minute Polygonum perfoliatum V 10. Clematis Clematis terniflora S 11. Privet Several species S 12. European sweetflag Acorus calamus H 13. Wineberry Rubus phoenicolasius S 14. Bamboo Several species H Restricted and Invasive 15. Japanese barberry Berberis thunbergii S 16. Periwinkle Vinca minor V 17. Garlic mustard Alliaria petiolata H 18. Winged euonymus Euonymus alata S 19. Porcelainberry Ampelopsis brevipedunculata V 20. Bradford pear Pyrus calleryana T 21. Marsh dewflower Murdannia keisak H 22. Lesser celandine Ranunculus ficaria H 23. Purple loosestrife Lythrum salicaria H 24. Reed canarygrass Phalaris arundinacea H 25. Honeysuckle Lonicera species S 26. Tree of heaven Alianthus altissima T 27. Spotted knapweed Centaruea biebersteinii H Restricted and Potentially-Invasive 28. Butterfly bush Buddleia davidii S Growth Habit: S=shrub, V=vine, H=herbaceous, T=tree, A=aquatic THE LIST • Plants on The List are non-native to Delaware, have the potential for widespread dispersal and establishment, can out-compete other species in the same area, and have the potential for rapid growth, high seed or propagule production, and establishment in natural areas. • Plants on Delaware’s Invasive Plant List were chosen by a committee of experts in environmental science and botany, as well as representatives of State agencies and the Nursery and Landscape Industry. -

Apiaceae (Pimp
Živné rostliny Druh vlnovníka, jeho bionomie Poznámka Živné rostliny polyfágních druhů Miříkovité – Apiaceae T Eriophyes peucedani (Pimpinella, Selinum aj.) Brukvovité – Brassicaceae T Aceria drabae (Cardaria, Capsella, Lepidium aj.) Lipnicovité – Poaceae K Aceria tenuis (Avena, Bromus, Dactylis aj.) Živné rostliny ostatních (steno- a monofágních) druhů jedle K Trisetacus floricolus (Abies) javor E Aceria carinifex, P Aceria cephalonea, O Aceria heteronyx, (Acer) P Aceria macrochela, E Aceria macrocheluserinea, P Aceria macrorhyncha, P Aceria myriadeum, E Aceria platanoidea, E Aceria pseudoplatani, A Aceria vermicularis, E Aculops aceris, P Aculops acericola, E Cecidophyes gymnaspis řebříček T Aceria kiefferi (Achillea) jírovec E Aculus hippocastani (Aesculus) zběhovec T Aceria ajugae (Ajuga) česnek N Aceria tulipae (Allium) olše E Acalitus brevitarsus, P Acalitus phyllereus, (Alnus) P Acaricalus trinotus, T Aceria longirostris, E Aceria nalepai, P Eriophyes alniincanae, E Eriophyes euryporus, P Eriophyes inangulis, P Eriophyes laevis pilát K Anthocoptes aspidophorus (Anchusa) pelyněk T Aceria artemisiae, T Aceria horrida, T Aceria subtilis (Artemisia) bukvice T Aceria solida (Betonica) bříza V Acalitus calycophthirus, E Acalitus longisetosus, (Betula) P Acalitus notolius, E Acalitus rudis, P Cecidophyopsis betulae, E Eriophyes leionotus, P Eriophyes lissonotus sveřep K Aculodes dubius (Bromus) zimostráz V Eriophyes canestrinii, P Eriophyes hypophyllus (Buxus) zvonek P Aceria campanulae, T Aculus schmardae (Campanula) habr E Aceria tenella, -

A New Species in the Genus Phyllocoptes Nalepa (Eriophyidae) from Greenhouse Roses in Poland
Acarologia A quarterly journal of acarology, since 1959 Publishing on all aspects of the Acari All information: http://www1.montpellier.inra.fr/CBGP/acarologia/ [email protected] Acarologia is proudly non-profit, with no page charges and free open access Please help us maintain this system by encouraging your institutes to subscribe to the print version of the journal and by sending us your high quality research on the Acari. Subscriptions: Year 2020 (Volume 60): 450 € http://www1.montpellier.inra.fr/CBGP/acarologia/subscribe.php Previous volumes (2010-2018): 250 € / year (4 issues) Acarologia, CBGP, CS 30016, 34988 MONTFERRIER-sur-LEZ Cedex, France ISSN 0044-586X (print), ISSN 2107-7207 (electronic) The digitalization of Acarologia papers prior to 2000 was supported by Agropolis Fondation under the reference ID 1500-024 through the « Investissements d’avenir » programme (Labex Agro: ANR-10-LABX-0001-01) Acarologia is under free license and distributed under the terms of the Creative Commons-BY-NC-ND which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited. Acarologia 56(2): 225–235 (2016) DOI: 10.1051/acarologia/20162236 A new species in the genus Phyllocoptes Nalepa (Eriophyidae) from greenhouse roses in Poland Tobiasz DRUCIAREK* and Mariusz LEWANDOWSKI (Received 27 August 2015; accepted 09 February 2016; published online 26 May 2016) Department of Applied Entomology, Faculty of Horticulture, Biotechnology and Landscape Architecture, Warsaw University of Life Sciences – SGGW. Nowoursynowska 159, 02-776 Warsaw, Poland. [email protected] (*Corresponding author), [email protected] ABSTRACT — A new eriophyid mite species in the Phyllocoptinae, namely Phyllocoptes resovius n. -

Eriophyoid Mite Fauna (Acari: Trombidiformes: Eriophyoidea) of Turkey: New Species, New Distribution Reports and an Updated Catalogue
Zootaxa 3991 (1): 001–063 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3991.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:AA47708E-6E3E-41D5-9DC3-E9D77EAB9C9E ZOOTAXA 3991 Eriophyoid mite fauna (Acari: Trombidiformes: Eriophyoidea) of Turkey: new species, new distribution reports and an updated catalogue EVSEL DENIZHAN1, ROSITA MONFREDA2, ENRICO DE LILLO2,4 & SULTAN ÇOBANOĞLU3 1Department of Plant Protection, Faculty of Agriculture, University of Yüzüncü Yıl, Van, Turkey. E-mail: [email protected] 2Department of Soil, Plant and Food Sciences (Di.S.S.P.A.), section of Entomology and Zoology, University of Bari Aldo Moro, via Amendola, 165/A, I–70126 Bari, Italy. E-mail: [email protected]; [email protected] 3Department of Plant Protection, Faculty of Agriculture, University of Ankara, Dıskapı, 06110 Ankara, Turkey. E-mail: [email protected] 4Corresponding author Magnolia Press Auckland, New Zealand Accepted by D. Knihinicki: 21 May 2015; published: 29 Jul. 2015 EVSEL DENIZHAN, ROSITA MONFREDA, ENRICO DE LILLO & SULTAN ÇOBANOĞLU Eriophyoid mite fauna (Acari: Trombidiformes: Eriophyoidea) of Turkey: new species, new distribution reports and an updated catalogue (Zootaxa 3991) 63 pp.; 30 cm. 29 Jul. 2015 ISBN 978-1-77557-751-5 (paperback) ISBN 978-1-77557-752-2 (Online edition) FIRST PUBLISHED IN 2015 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2015 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. -

Mistaken Identity? Invasive Plants and Their Native Look-Alikes: an Identification Guide for the Mid-Atlantic
Mistaken Identity ? Invasive Plants and their Native Look-alikes an Identification Guide for the Mid-Atlantic Matthew Sarver Amanda Treher Lenny Wilson Robert Naczi Faith B. Kuehn www.nrcs.usda.gov http://dda.delaware.gov www.dsu.edu www.dehort.org www.delawareinvasives.net Published by: Delaware Department Agriculture • November 2008 In collaboration with: Claude E. Phillips Herbarium at Delaware State University • Delaware Center for Horticulture Funded by: U.S. Department of Agriculture Natural Resources Conservation Service Cover Photos: Front: Aralia elata leaf (Inset, l-r: Aralia elata habit; Aralia spinosa infloresence, Aralia elata stem) Back: Aralia spinosa habit TABLE OF CONTENTS About this Guide ............................1 Introduction What Exactly is an Invasive Plant? ..................................................................................................................2 What Impacts do Invasives Have? ..................................................................................................................2 The Mid-Atlantic Invasive Flora......................................................................................................................3 Identification of Invasives ..............................................................................................................................4 You Can Make a Difference..............................................................................................................................5 Plant Profiles Trees Norway Maple vs. Sugar -

Determination of Taxonomic Status of Chinese Species of the Genus Clematis by Using High Performance Liquid Chromatography–Mass Spectrometry (Hplc-Ms) Technique
Pak. J. Bot., 42(2): 691-702, 2010. DETERMINATION OF TAXONOMIC STATUS OF CHINESE SPECIES OF THE GENUS CLEMATIS BY USING HIGH PERFORMANCE LIQUID CHROMATOGRAPHY–MASS SPECTROMETRY (HPLC-MS) TECHNIQUE MUHAMMAD ISHTIAQ CH1, 2*, Q. HE2, S. FENG, YI WANG2, P.G. XIAO2, YIYU CHENG2* AND HABIB AHMED3 1Department of Botany, Mirpur University of Science & Technology (MUST) Bhimber Campus, (AK) Pakistan 2Department of Chinese Medicine Science and Engineering, College of Pharmaceutical Sciences, Institute of Bioinformatics, Zhejiang University, No. 38 Zhe da Road Hangzhou, 310027, P. R. China 3Department of Botany, University of Hazara, Garden Campus Mansehra, NWFP, Pakistan. Abstract A comparative taxonomic study using chemometric and numerical taxonomic approaches on 15 populations of 12 species of major taxa of the genus Clematis, belonging to sections, Rectae, Clematis, Meclatis, Tubulosae and Viorna were analyzed by HPLC coupled with diode array detector and ESI-MS. The chemodiversity profile of saponins proved to be useful taxonomic markers for the genus and results are presented in phenograms. The compound ‘Huzhangoside D’ was the most abundant in analyzed species of the genus. Numerical taxonomic study was also conducted based on phylogenetically informative characters which corroborated with chemical fingerprinting findings. The significance of chemical markers in taxonomic study as well as their correlation between morphology and chemical compound profile is also debated with its significant role in botanic drugs identification. Introduction Clematis L., is the second largest genus of Ranunculaceae, which comprises of more than 300 species worldwide and among these 147 (93 endemic) species are found in China (Wang, 1999). Traditional classification systems are usually based on floral and vegetative characters, and have been used for taxonomic divisions of many plants. -

Acari Scientific Classification Kingdom
Acari - Wikipedia https://en.wikipedia.org/wiki/Acari From Wikipedia, the free encyclopedia Acari (or Acarina) are a taxon of arachnids that contains mites and ticks. The diversity of the Acari is extraordinary and its fossil history Acari goes back to at least the early Devonian period.[1] As a result, Temporal range: acarologists (the people who study mites and ticks) have proposed a Early Devonian–Recent complex set of taxonomic ranks to classify mites. In most modern treatments, the Acari is considered a subclass of Arachnida and is PreЄ Є O S D C P T J K Pg N composed of two or three superorders or orders: Acariformes (or Actinotrichida), Parasitiformes (or Anactinotrichida), and Opilioacariformes; the latter is often considered a subgroup within the Parasitiformes. The monophyly of the Acari is open to debate, and the relationships of the acarines to other arachnids is not at all clear.[2] In older treatments, the subgroups of the Acarina were placed at order rank, but as their own subdivisions have become better-understood, it is more usual to treat them at superorder rank. Most acarines are minute to small (e.g., 0.08–1.00 millimetre or 0.003–0.039 inches), but the largest Acari (some ticks and red velvet mites) may reach lengths of 10–20 millimetres (0.4–0.8 in). Over 50,000 species have been described (as of 1999) and it is estimated that a million or more species may exist. The study of mites and ticks is called acarology (from Greek ἀκαρί/ἄκαρι, akari, a type of mite; and -λογία, -logia),[3] and the leading scientific journals for acarology include Acarologia, Experimental and Applied Acarology and the Peacock mite (Tuckerella sp.), International Journal of Acarology. -

Biology and Behavior of the Mite Cheletomorpha Lepidopterorum (Shaw) (Prostigmata:Cheyletidae) and Its Role As a Predator of a Grain Mite Acarus Farris (Oud
AN ABSTRACT OF THE THESIS OF JAMES ROGER ALLISONfor the DOCTOR OF PHILOSOPHY (Name (Degree) in ENTOMOLOGY presented on41a21712Ajd2W;) /2.'7/ (Major) (Date) Title: BIOLOGY AND BEHAVIOR OF THE MITECHELETOMORPHA LEPIDOPTERORUM (SHAW) (PROSTIGMATA:CHEYLETIDAE) AND ITS ROLE AS A PREDATOR OF A GRAIN MITEACARUS FARRIS (OUD. )(ASTIGIV&TIAaR. Redacted for Privacy Abstract approved: /7J //I G.- W. Krantz Cheletomorpha lepidopterorum (Shaw), a predaceous, prostig- matid mite, was studied under laboratory conditions of20° - 30° C and 80% - 90% R. H. to determine its effectiveness as apossible biological control agent of Acarus farris (Oud. ),a graminivorous mite which infests stored grains and grain products.Although Cheletophyes knowltoni Beer and Dailey had been synonymized with C. lepidopterorum, it was found that the latter couldbe differentiated from C. knowltoni on the basis of biological, morphological,and behavioral data obtained from four species "populations"(Kansas, Oregon, California, and World-Wide). A temperature range of 20° - 25° C and relative humidities of 80% - 90% created conditions ideally suited to the rearing 'of C. lepidopterorum.Egg survival under optimal temperature and humidity regimes exceeded75%. Mated females laid more eggs than unmatedfemales at optimal environmental conditions. Development time from egg to adult ranged from alow of 192 hours for a single male at 30° C, 90% R. H. ,to 420 hours for a male at 20° C, 90% R. H.The second nymphal stage sometimes was omitted in the male ontogeny. Mated females produced male and female progeny,while unmated females produced a higher percentage ofmales. Starved C. lepidopterorum females survivedlongest at 20° C, 80% R. H. -- 31. -

Vascular Plant Inventory and Ecological Community Classification for Cumberland Gap National Historical Park
VASCULAR PLANT INVENTORY AND ECOLOGICAL COMMUNITY CLASSIFICATION FOR CUMBERLAND GAP NATIONAL HISTORICAL PARK Report for the Vertebrate and Vascular Plant Inventories: Appalachian Highlands and Cumberland/Piedmont Networks Prepared by NatureServe for the National Park Service Southeast Regional Office March 2006 NatureServe is a non-profit organization providing the scientific knowledge that forms the basis for effective conservation action. Citation: Rickie D. White, Jr. 2006. Vascular Plant Inventory and Ecological Community Classification for Cumberland Gap National Historical Park. Durham, North Carolina: NatureServe. © 2006 NatureServe NatureServe 6114 Fayetteville Road, Suite 109 Durham, NC 27713 919-484-7857 International Headquarters 1101 Wilson Boulevard, 15th Floor Arlington, Virginia 22209 www.natureserve.org National Park Service Southeast Regional Office Atlanta Federal Center 1924 Building 100 Alabama Street, S.W. Atlanta, GA 30303 The view and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government. This report consists of the main report along with a series of appendices with information about the plants and plant (ecological) communities found at the site. Electronic files have been provided to the National Park Service in addition to hard copies. Current information on all communities described here can be found on NatureServe Explorer at www.natureserveexplorer.org. Cover photo: Red cedar snag above White Rocks at Cumberland Gap National Historical Park. Photo by Rickie White. ii Acknowledgments I wish to thank all park employees, co-workers, volunteers, and academics who helped with aspects of the preparation, field work, specimen identification, and report writing for this project. -

Morphology and Biology of Phyllocoptes Azadirachtae Chandrapatya (Acari : Eriophyidae)
Kasetsart J. (Nat. Sci.) 38 : 475 - 483 (2004) Morphology and Biology of Phyllocoptes azadirachtae Chandrapatya (Acari : Eriophyidae) Pavinee Noochanapai and Angsumarn Chandrapatya ABSTRACT Phyllocoptes azadirachtae Chandrapatya belongs to Family Eriophyidae, Suborder Actinedida. The scanning electron photographs of dorsal shield, genitalia, microtubercle and featherclaw are presented. This mite is able to feed and reproduce on 3 neem plants; Azadirachta indica Juss. siamensis Val. (Sadao-Thai), Azadirachta indica Juss. (Sadao-India) and Azadirachta excelsa (Jack) Jacobs (Sadao-Chang). Sadao-Thai is proved to be the most suitable host for P. azadirachtae judging from low mortality rate during development (7%), short life cycle (7.8 days) and high fecundity (21.8 eggs/female). Key words: Phyllocoptes azadirachtae, neem, eriophyid mite, morphology, biology INTRODUCTION In Thailand, D. azadirachtae was reported as a vagrant mite where P. azadirachtae caused Eriophyoids are the smallest phytophagous russetting on the lower leaf surface (Boczek and mites, ranging in length from 80 to 300 µm. Most Chandrapatya, 1992, 1993). Sombatsiri et al. eriophyoids are host specific, causing galls, erinea, (1995) reported that young neem leaves infested russetting and leaf or shoot deformation, and many by eriophyoid mites might become malformed and species are leaf vagrants (Keifer, 1952; Jepson et dried up while old leaflets became yellowish and al., 1975; Chandrapatya and Baker, 1986). Three dropped. Preliminary surveys of the neem mite in species of eriophyoid mite were recorded feeding several parts of Thailand revealed that P. on Sadao India (Azadirachta indica Juss.) namely azadirachtae was commonly found on 3 varieties Phyllocoptes azadirachtae Chandrapatya, of neem, Azadirachta indica Juss. siamensis Val.