GSK Annual Report 2005 01 However, Addressing This Challenge Is Something GSK Cannot “The Tragedies During the Year Brought Home to Me the Extent Do Alone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
View/Download
Solutions for Affordable Rental Housing in the Delaware Valley r-- - 0 0 0 DDD DDD /-""" D D •• DELAWARE VALLEY' REGIONAL PLANNING COMMISSION va August 1994 The preparation of this report was funded through federal grants from the U. S. Department of Transportation's Federal Highway Administration (FHW A) and Federal Transit Administration (FTA), the Pennsylvania and New Jersey Departments of Transportation as well as by DVRPC's state and local member governments. The authors, however, are solely responsible for its [mdings and conclusions, which may not represent the official views or policies of the funding agencies. Created in 1965, the Delaware Valley Regional Planning Commission (DVRPC) is an interstate, intercounty and intercity agency which provides continuing, comprehensive and coordinated planning for the orderly growth and development of the Delaware Valley region. The region includes Bucks, Chester, Delaware, and Montgomery counties as well as the City of Philadelphia in Pennsylvania and Burlington, Camden, Gloucester, and Mercer counties in New Jersey. The Commission is an advisory agency which divides its planning and service functions between the Office of the Executive Director, the Office of Public Affairs, and three line Divisions: Transportation Planning, Regional Planning, and Administration. DVRPC's mission for the 1990s is to emphasize technical assistance and services and to conduct high priority studies for member state and local governments, while determining and meeting the needs of the private sector. The DVRPC logo is adapted from the official seal of the Commission and is designed as a stylized image of the Delaware Valley. The outer ring symbolizes the region as a whole while the diagonal bar signifies the Delaware River flowing through it. -

Corporate Governance
Strategic report Governance and remuneration Financial statements Investor information Corporate Governance In this section Chairman’s Governance statement 78 The Board 80 Corporate Executive Team 83 Board architecture 85 Board roles and responsibilities 86 Board activity and principal decisions 87 Our purpose, values and culture 90 The Board’s approach to engagement 91 Board performance 94 Board Committee information 96 Our Board Committee reports 97 Section 172 statement 108 Directors’ report 109 GSK Annual Report 2020 77 Chairman’s Governance statement In last year’s Governance statement, I explained that our primary Education and focus on Science objective for 2020 was to ensure there was clarity between the Given the critical importance of strengthening the pipeline, Board and management on GSK’s execution of strategy and its the Board has benefitted from devoting a higher proportion of operational priorities. We have aligned our long-term priorities its time in understanding the science behind our strategy and of Innovation, Performance and Trust powered by culture testing its application. It is important that the Board has a and agreed on the metrics to measure delivery against them. working understanding of the key strategic themes upon The Board’s annual cycle of meetings ensures that all major which our R&D strategy is based. These themes have been components of our strategy are reviewed over the course complemented by Board R&D science thematic deep dives. of the year. Our focus was on the fundamentals of our strategy: human The COVID-19 pandemic impacted and dominated all our genetics, the immune system and AI and ML, as well as to lives for the majority of 2020. -

PEDIARIX Is a Vaccine
HIGHLIGHTS OF PRESCRIBING INFORMATION • If Guillain-Barré syndrome occurs within 6 weeks of receipt of a prior These highlights do not include all the information needed to use vaccine containing tetanus toxoid, the decision to give PEDIARIX should PEDIARIX safely and effectively. See full prescribing information for be based on potential benefits and risks. (5.2) PEDIARIX. • The tip caps of the prefilled syringes contain natural rubber latex which may cause allergic reactions. (5.3) PEDIARIX [Diphtheria and Tetanus Toxoids and Acellular Pertussis • Syncope (fainting) can occur in association with administration of Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus injectable vaccines, including PEDIARIX. Procedures should be in place Vaccine], Suspension for Intramuscular Injection to avoid falling injury and to restore cerebral perfusion following Initial U.S. Approval: 2002 syncope. (5.4) • If temperature ≥105°F, collapse or shock-like state, or persistent, ----------------------------- INDICATIONS AND USAGE ---------------------------- PEDIARIX is a vaccine indicated for active immunization against diphtheria, inconsolable crying lasting ≥3 hours have occurred within 48 hours after tetanus, pertussis, infection caused by all known subtypes of hepatitis B virus, receipt of a pertussis-containing vaccine, or if seizures have occurred and poliomyelitis. PEDIARIX is approved for use as a 3-dose series in infants within 3 days after receipt of a pertussis-containing vaccine, the decision born of hepatitis B surface antigen (HBsAg)-negative mothers. PEDIARIX to give PEDIARIX should be based on potential benefits and risks. (5.5) may be given as early as 6 weeks of age through 6 years of age (prior to the • For children at higher risk for seizures, an antipyretic may be 7th birthday). -

Final Attendee List
Jennifer Abnet GlaxoSmithKline I GlaxoSmithKline I Jane Arboleda APRN Guardant I Molly Benson Sanjiv Agarwala MD Puma Biotechnology I Cancer Expert Now I Joe Arminger AbbVie I Seth Berkowitz LCSW, Manmeet Ahluwalia MD, CCLS MBA Patricia Armstrong The Leukemia & Lymphoma Miami Cancer Institute I Novartis I Society V Steve Albers Kim Arnold APRN CPNP Barry Berman MD, MS Alexion V CPHON Florida Cancer Specialists I Servier Pharmaceuticals I Maritza Alencar DNP, Tizano Bernard MBA, APRN, BMTCN Sheila Arrington MSN, Cancer Care Centers of Miami Cancer Institute V APRN, NP-C, AOCN Brevard I Puma Biotechnology I Carmen Allen MSIT Ana Mari Bernardini Pharmavoxx V Shannon Ashmon Novartis Oncology I Eisai, Inc I Luly Almeida Bernard Berry MBA Incyte Corporation I Sarah Ashton MS AstraZeneca Pharmaceuticals Guardant Health I I Talat Almukhtar MD Orlando Health Cancer Melissa Austin Jason Bever Institute I Cancer Care Centers of Oncopeptides I Brevard I Beatrice Alvarado Roberts Amy Bignon MD Garland Avera Doyle Caris Life Sciences I University of Florida I Jazz Pharmaceuticals I Nadeem Bilani MD Tadeu Ambros MD Francie Babcock Cleveland Clinic I FCS I AMAG Pharmaceuticals/Covis I Angela Bilik RN BSN Douglas Anderson AstraZeneca I Incyte Corporation I Craig Bailey Astra Zeneca I Jamie Bilsky BS Blesson Andrews Genentech V Genentech I Kevin Barr Daiichi Sankyo I Rohit Bishnoi MD Ollie Annum PharmD University of Florida I BHMCR I Leonard Bennett PharmD EMD Serono I Brady Blackman Susmitha Apuri MD MorphoSys I Florida Cancer Specialists I Michael Bennett As of 4-22-21 Kimberly Blandon RN MSN Rick Breitenstein Memorial Cancer Institute I Apellis Pharmaceuticals I Denise Capo Karyopharm Therapeutics I Taryn Boiteau GlaxoSmithKline I Amanda Bridges Florida Society of Clinical Heidi Caravetta Tracy Bonds RN, BSN, Oncology I Exelixis, Inc. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -
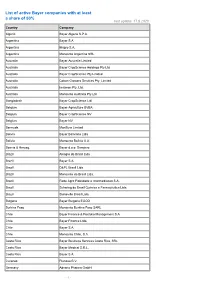
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

The Korn/Ferry Market Cap 100 Board Leadership at America’S Most Valuable Public Companies About the 2010 Korn/Ferry Market Cap 100
The Korn/Ferry Market Cap 100 Board leadership at America’s most valuable public companies About the 2010 Korn/Ferry Market Cap 100 The Korn/Ferry Market Cap 100 (KFMC100) comprises the U.S. companies traded on the NYSE or NASDAQ , excluding public investment firms, with the largest market capitalization as of the close of market on May 3, 2010, after reporting for the 2009 fiscal year. Data about the companies’ boards, compiled from proxies related to fiscal year 2009, provide the baseline for this report. B Contents Introduction . 2 Board leadership in an era of change . 4 What’s in a name? . 4 Portrait of a lead director . 6 Interview with Mark Ketchum . 8 Lead director, Kraft Foods Adding value to the board . 12 Interview with Irv Hockaday . 14 Presiding director, Ford Motor Co. and Estée Lauder Companies Inc. Committee membership and leadership . 16 Leadership succession on the board . 18 Assessing the need for terms . 19 Interview with Bonnie G. Hill . 20 Lead director, The Home Depot, Inc. Compensating for time and value . 22 Final thoughts and best practices . 24 Appendix A . 25 Board data Appendix B . 32 List of the KFMC100 companies Appendix C . 35 List of the KFMC100 non-executive board leaders Introducing the Korn/Ferry Market Cap 100 The Korn/Ferry Market Cap 100 (KFMC100) marks a new approach to the governance research our firm has undertaken for thirty-five years. We’ve chosen to examine the United States’ one hundred most valuable compa- nies, working from the assumption that leadership models represented on these boards are worth observing and emulating. -
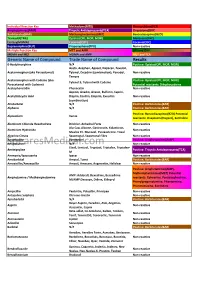
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

Steven Sanders, Et Al. V. Bayer (AG) Aktiengesellschaft, Et Al. 03-CV-1546-Second Consolidated Amended Complaint
Q,^.IGiNAL &&,iN 2i 4- L UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF NEW YORK -_DOF x Consolidated Civil Action IN RE BAYER AG SECURITIES No. 03 CV 1546 (WHP) LITIGATION Class Action x 71 :n = SECOND CONSOLIDATED AMENDED COMPLAINT Jury Trial Demand MILBERG WEISS BERSHAD & SCHULMAN LLP Melvyn I. Weiss (MW-1392) Michael C . Spencer (MS-8874) Lee A. Weiss (LW-1130) Jennifer Sclar (JS-7313) One Pennsylvania Plaza New York, NY 10119 (212) 594-5300 Attorneys for Lead Plaintiff January 14, 2005 i • ' Table of Contents Page SUMMARY OF CLAIMS ...............................................................................................................2 JURISDICTION AND VENUE ...................................................................................................... 8 A. General Jurisdiction and Venue ............................................................................... 8 B. Subject Matter Jurisdiction Over Claims of Foreign Purchasers on Foreign Exchanges ................................................................................................................ 8 PARTIES .......................................................................................................................................12 RELEVANT EVENTS ..................................................................................................................14 A. Background of the Statin Market ...........................................................................14 B. FDA Approval and Introduction of Baycol; Early Safety Warnings -

GSK Q1 Report 2021
Calibrating Precision GSK Pakistan Limited FirstAnnual Quarter Report Report 20212020 CORPORATE INFORMATION As at March 31, 2021 Board of Directors Management Committee Disclosure Committee Mr. Dmytro Oliinyk Ms. Erum Shakir Rahim Ms. Erum Shakir Rahim Chairman Chief Executive Officer Chairperson Ms. Erum Shakir Rahim Mr. Abdul Samad* Mr. Abdul Samad* Chief Executive Officer Chief Financial Officer Member Mr. Abdul Samad* Mehar-e-daraksha Ameer Mr. Dmytro Oliinyk Chief Financial Officer Legal Director Member Ms. Maheen Rahman Dr. Tariq Farooq Mehar-e-daraksha Ameer Independent Director Business Unit Director BU 1 Member Mr. Muneer Kamal Syed Nasir Farid** Company Secretary Independent Director Business Unit Director BU 2 Ms. Mehar-e-daraksha Ameer Mr. Mehmood Mandviwalla Dr. Naved Masoom Ali Non-Executive Director Business Unit Director BU 3 Chief Financial Officer Mr. Mark Dawson Khurram Amjad Mr. Abdul Samad* Non-Executive Director Director Commercial Chief Internal Auditor Excellence & CTC Audit Committee Syed Ahsan Ejaz Dr. Gohar Nayab Khan Mr. Muneer Kamal Regulatory Affairs Cluster Bankers Chairman Head - Pakistan & Iran Citibank NA Mr. Dmytro Oliinyk Mr. Abdul Haseeb Pirzada Deutsche Bank A.G. Member Director Corporate Affairs and Habib Bank Limited Administration Meezan Bank Limited Mr. Mark Dawson Standard Chartered Bank (Pakistan) Ltd Member Mr. Zain Anjum Country Compliance Officer Auditors Mr. Mehmood Mandviwalla Member Dr. Yousuf Hasan Khan Yousuf Adil & Co. Chartered Director Medical Accountants Ms. Maheen Rahman Member Syed Nabigh Raza Alam Legal Advisors Tech Head Hashmi & Hashmi Human Resource Mr. Obaid Siddiqui Faisal, Mahmood Ghani and Co & Remuneration Head of Procurement Legal Consultancy Inc. Committee Mr. Farqaleet Iqbal Registered Office Mr. -

2005 GSK Corporate Responsibility Report
2005 GSK Corporate Responsibility Report Introduction 1 Introduction Page Our contribution to society 1 About this report 1 CEO and Chairman’s letter 3 Managing corporate responsibility 4 Why CR is important to GSK 4 CR governance 4 Our CR principles 6 Stakeholder engagement 7 Stakeholder feedback 7 How we are responding 9 Government and external affairs 10 Membership of trade associations 10 US lobbying expenditures 10 Political donations 10 Our position on key issues 11 Patient advocacy 11 2 Access to medicine 3 Research 4 Ethical conduct 5 Employment practices 6 Human rights 7 Environment 8 Community investment 9 Data summary 1 1 GSK CORPORATE RESPONSIBILITY REPORT 2005 Introduction Welcome to GSK’s Corporate Responsibility Report 2005. This report explains our approach to the wide range of social, ethical and environmental issues associated with our business and reports our performance in 2005. The full web-based report is available on www.gsk.com GSK is a research-based pharmaceutical company, with operations in 119 countries. We make prescription medicines, vaccines, over-the-counter medicines, and consumer healthcare products. Our business accounts for 6.3% of the world’s pharmaceutical market. We have strong positions in several therapeutic areas including anti-infectives, asthma, cancer, cardiovascular, depression, diabetes, HIV/AIDS and urology. For an overview of our business, see our Annual Report. OUR CONTRIBUTION TO SOCIETY This report explains our approach to the significant In the last 80 years, medicines and vaccines have corporate responsibility issues for our business, including: transformed millions of lives. They have helped to increase • Access to medicines – how we make our medicines life expectancy and lowered death rates from conditions accessible to poor patients in developed and developing such as heart disease, stroke and cancer.