4411784.Pdf (302.2Kb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Overview of the Kallikrein Gene Families in Humans and Other Species: Emerging Candidate Tumour Markers૾
Clinical Biochemistry 36 (2003) 443–452 An overview of the kallikrein gene families in humans and other species: Emerging candidate tumour markers૾ George M. Yousefa,b, Eleftherios P. Diamandisa,b,* aDepartment of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, Ontario, Canada bDepartment of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada Abstract Kallikreins are serine proteases with diverse physiologic functions. They are represented by multigene families in many animal species, especially in rat and mouse. Recently, the human kallikrein gene family has been fully characterized and includes 15 members, tandemly localized on chromosome 19q13.4. A new definition has now been proposed for kallikreins, which is not based on function but, rather, on close proximity and structural similarities. In this review, we summarize available information about kallikreins in many animal species with special emphasis on human kallikreins. We discuss the common structural features of kallikreins at the DNA, mRNA and protein levels and overview their evolutionary history. Kallikreins are expressed in a wide range of tissues including the salivary gland, endocrine or endocrine-related tissues such as testis, prostate, breast and endometrium and in the central nervous system. Most, if not all, genes are under steroid hormone regulation. Accumulating evidence indicates that kallikreins are involved in many pathologic conditions. Of special interest is the potential role of kallikreins in the central nervous system. In addition, many kallikreins seem to be candidate tumor markers for many malignancies, especially those of endocrine-related organs. © 2003 The Canadian Society of Clinical Chemists. All rights reserved. Keywords: Kallikrein; Tumor markers; Cancer biomarkers; Prostate cancer; Breast cancer; Ovarian cancer; Alzheimer’s disease; Serine proteases; Chromosome 19; Kallikrein evolution; Rodent kallikreins; Hormonally regulated genes 1. -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Download, Or Email Articles for Individual Use
Florida State University Libraries Faculty Publications The Department of Biomedical Sciences 2010 Functional Intersection of the Kallikrein- Related Peptidases (KLKs) and Thrombostasis Axis Michael Blaber, Hyesook Yoon, Maria Juliano, Isobel Scarisbrick, and Sachiko Blaber Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] Article in press - uncorrected proof Biol. Chem., Vol. 391, pp. 311–320, April 2010 • Copyright ᮊ by Walter de Gruyter • Berlin • New York. DOI 10.1515/BC.2010.024 Review Functional intersection of the kallikrein-related peptidases (KLKs) and thrombostasis axis Michael Blaber1,*, Hyesook Yoon1, Maria A. locus (Gan et al., 2000; Harvey et al., 2000; Yousef et al., Juliano2, Isobel A. Scarisbrick3 and Sachiko I. 2000), as well as the adoption of a commonly accepted Blaber1 nomenclature (Lundwall et al., 2006), resolved these two fundamental issues. The vast body of work has associated 1 Department of Biomedical Sciences, Florida State several cancer pathologies with differential regulation or University, Tallahassee, FL 32306-4300, USA expression of individual members of the KLK family, and 2 Department of Biophysics, Escola Paulista de Medicina, has served to elevate the importance of the KLKs in serious Universidade Federal de Sao Paulo, Rua Tres de Maio 100, human disease and their diagnosis (Diamandis et al., 2000; 04044-20 Sao Paulo, Brazil Diamandis and Yousef, 2001; Yousef and Diamandis, 2001, 3 Program for Molecular Neuroscience and Departments of 2003; -
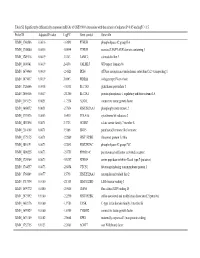
Table SI. Significantly Differentially Expressed Mrnas of GSE19089 Data Series with the Criteria of Adjusted P<0.05 Andlogfc
Table SI. Significantly differentially expressed mRNAs of GSE19089 data series with the criteria of adjusted P<0.05 andlogFC>1.5. Probe ID Adjusted P-value LogFC Gene symbol Gene title ILMN_1740586 0.0016 -3.0593 PTHLH phospholipase A2 group IIA ILMN_1705080 0.0016 -5.0044 PTHLH secreted LY6/PLAUR domain containing 1 ILMN_1749118 0.0019 3.1363 LAMC2 calmodulin like 5 ILMN_1800341 0.0019 2.4138 CALML5 WD repeat domain 66 ILMN_1674460 0.0019 -2.4821 IRX4 ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 1 ILMN_1675857 0.0019 2.0803 WDR66 collagen type IV 6 chain ILMN_1726666 0.0038 -3.8331 SLC7A8 glutathione peroxidase 3 ILMN_2056606 0.0047 -2.1260 SLC2A1 protein phosphatase 1 regulatory inhibitor subunit 1A ILMN_2115125 0.0051 -1.7534 ALG1L connective tissue growth factor ILMN_1668072 0.0051 -2.7854 HIST2H2AA3 phosphoglycerate mutase 2 ILMN_1739576 0.0053 1.6410 COL4A6 cytochrome b5 reductase 2 ILMN_1807894 0.0071 2.3723 HOXB7 solute carrier family 7 member 8 ILMN_2314169 0.0071 3.5186 ISG15 parathyroid hormone like hormone ILMN_1775175 0.0071 -2.2869 HIST1H2BK ribosomal protein L3 like ILMN_1810191 0.0073 -2.2843 HIST2H2AC phospholipase A2 group IVC ILMN_1800225 0.0073 -2.0751 PPP1R14C peroxisome proliferator activated receptor ILMN_1720984 0.0073 -5.5257 NDRG4 serine peptidase inhibitor Kazal type 7 (putative) ILMN_1764557 0.0073 -2.6854 VTCN1 fat storage inducing transmembrane protein 1 ILMN_1760849 0.0077 1.5798 HIST2H2AA3 neuropilin and tolloid like 2 ILMN_1713534 0.0100 -2.1515 HIST1H2BD LIM domain binding 3 ILMN_1699772 -

Activation Profiles and Regulatory Cascades of the Human Kallikrein-Related Peptidases Hyesook Yoon
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2008 Activation Profiles and Regulatory Cascades of the Human Kallikrein-Related Peptidases Hyesook Yoon Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES ACTIVATION PROFILES AND REGULATORY CASCADES OF THE HUMAN KALLIKREIN-RELATED PEPTIDASES By HYESOOK YOON A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Fall Semester, 2008 The members of the Committee approve the dissertation of Hyesook Yoon defended on July 10th, 2008. ________________________ Michael Blaber Professor Directing Dissertation ________________________ Hengli Tang Outside Committee Member ________________________ Brian Miller Committee Member ________________________ Oliver Steinbock Committee Member Approved: ____________________________________________________________ Joseph B. Schlenoff, Chair, Department of Chemistry and Biochemistry The Office of Graduate Studies has verified and approved the above named committee members. ii ACKNOWLEDGMENTS I would like to dedicate this dissertation to my parents for all your support, and my sister and brother. I would also like to give great thank my advisor, Dr. Blaber for his patience, guidance. Without him, I could never make this achievement. I would like to thank to all the members in Blaber lab. They are just like family to me and I deeply appreciate their kindness, consideration and supports. I specially like to thank to Mrs. Sachiko Blaber for her endless guidance and encouragement. I would like to thank Dr Jihun Lee, Margaret Seavy, Rani and Doris Terry for helpful discussions and supports. -

A Multiparametric Serum Kallikrein Panel for Diagnosis of Non ^ Small
Imaging, Diagnosis, Prognosis A Multiparametric Serum Kallikrein Panel for Diagnosis of Non ^ Small Cell Lung Carcinoma Chris Planque,1, 2 Lin Li,3 Yingye Zheng,3 Antoninus Soosaipillai,1, 2 Karen Reckamp,4 David Chia,5 Eleftherios P. Diamandis,1, 2 and Lee Goodglick5 Abstract Purpose: Human tissue kallikreins are a family of15 secreted serine proteases.We have previous- ly shown that the expression of several tissue kallikreins is significantly altered at the transcription- al level in lung cancer. Here, we examined the clinical value of 11members of the tissue kallikrein family as potential biomarkers for lung cancer diagnosis. Experimental Design: Serum specimens from 51 patients with non ^ small cell lung cancer (NSCLC) and from 50 healthy volunteers were collected. Samples were analyzed for11kallikreins (KLK1, KLK4-8, and KLK10-14) by specific ELISA. Data were statistically compared and receiver operating characteristic curves were constructed for each kallikrein and for various combinations. Results: Compared with sera from normal subjects, sera of patients with NSCLC had lower levels of KLK5, KLK7, KLK8, KLK10, and KLK12, and higher levels of KLK11, KLK13, and KLK14. Expres- sion of KLK11and KLK12 was positively correlated with stage.With the exception of KLK5, expres- sion of kallikreins was independent of smoking status and gender. KLK11, KLK12, KLK13, and KLK14 were associated with higher risk of NSCLC as determined by univariate analysis and con- firmed by multivariate analysis.The receiver operating characteristic curve of KLK4, KLK8, KLK10, KLK11,KLK12, KLK13, and KLK14 combined exhibited an area under the curve of 0.90 (95% con- fidence interval, 0.87-0.97). -

A Genomic Analysis of Rat Proteases and Protease Inhibitors
A genomic analysis of rat proteases and protease inhibitors Xose S. Puente and Carlos López-Otín Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología, Universidad de Oviedo, 33006-Oviedo, Spain Send correspondence to: Carlos López-Otín Departamento de Bioquímica y Biología Molecular Facultad de Medicina, Universidad de Oviedo 33006 Oviedo-SPAIN Tel. 34-985-104201; Fax: 34-985-103564 E-mail: [email protected] Proteases perform fundamental roles in multiple biological processes and are associated with a growing number of pathological conditions that involve abnormal or deficient functions of these enzymes. The availability of the rat genome sequence has opened the possibility to perform a global analysis of the complete protease repertoire or degradome of this model organism. The rat degradome consists of at least 626 proteases and homologs, which are distributed into five catalytic classes: 24 aspartic, 160 cysteine, 192 metallo, 221 serine, and 29 threonine proteases. Overall, this distribution is similar to that of the mouse degradome, but significatively more complex than that corresponding to the human degradome composed of 561 proteases and homologs. This increased complexity of the rat protease complement mainly derives from the expansion of several gene families including placental cathepsins, testases, kallikreins and hematopoietic serine proteases, involved in reproductive or immunological functions. These protease families have also evolved differently in the rat and mouse genomes and may contribute to explain some functional differences between these two closely related species. Likewise, genomic analysis of rat protease inhibitors has shown some differences with the mouse protease inhibitor complement and the marked expansion of families of cysteine and serine protease inhibitors in rat and mouse with respect to human. -

Cloud-Clone Productlist Viroquest Inc
Cloud-Clone ProductList ViroQuest Inc. Product No. Name Format CEA544Hu ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate CEB409Hu ELISA Kit for Hemoglobin (HB) 96-well strip plate CEB028Ra ELISA Kit for Albumin (ALB) 96-well strip plate SEB409Mu ELISA Kit for Hemoglobin (HB) 96-well strip plate CEA544Gu ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate SEB409Rb ELISA Kit for Hemoglobin (HB) 96-well strip plate CEB028Rb ELISA Kit for Albumin (ALB) 96-well strip plate SEB409Ra ELISA Kit for Hemoglobin (HB) 96-well strip plate CEB028Gu ELISA Kit for Albumin (ALB) 96-well strip plate HEA544Hu High Sensitive ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate CEA544Mu ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate CEB028Mu ELISA Kit for Albumin (ALB) 96-well strip plate CEC036Mu ELISA Kit for Transferrin (TRF) 96-well strip plate CEA544Ga ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate CEA544Ra ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate CEA544Rb ELISA Kit for Immunoglobulin G (IgG) 96-well strip plate SEB409Gu ELISA Kit for Hemoglobin (HB) 96-well strip plate SEA448Hu ELISA Kit for Insulin (INS) 96-well strip plate SEA480Hu ELISA Kit for Myoglobin (MYO) 96-well strip plate SEA518Hu ELISA Kit for Ferritin (FE) 96-well strip plate SEC036Ca ELISA Kit for Transferrin (TRF) 96-well strip plate CEB028Hu ELISA Kit for Albumin (ALB) 96-well strip plate CEB028Ca ELISA Kit for Albumin (ALB) 96-well strip plate SEB409Eq ELISA Kit for Hemoglobin (HB) 96-well strip plate SEA049Hu ELISA Kit for Interferon -

Human KLK12 ELISA Kit (ARG81514)
Product datasheet [email protected] ARG81514 Package: 96 wells Human KLK12 ELISA Kit Store at: 4°C Component Cat. No. Component Name Package Temp ARG81514-001 Antibody-coated 8 X 12 strips 4°C. Unused strips microplate should be sealed tightly in the air-tight pouch. ARG81514-002 Standard 2 X 10 ng/vial 4°C ARG81514-003 Standard/Sample 30 ml (Ready to use) 4°C diluent ARG81514-004 Antibody conjugate 1 vial (100 µl) 4°C concentrate (100X) ARG81514-005 Antibody diluent 12 ml (Ready to use) 4°C buffer ARG81514-006 HRP-Streptavidin 1 vial (100 µl) 4°C concentrate (100X) ARG81514-007 HRP-Streptavidin 12 ml (Ready to use) 4°C diluent buffer ARG81514-008 25X Wash buffer 20 ml 4°C ARG81514-009 TMB substrate 10 ml (Ready to use) 4°C (Protect from light) ARG81514-010 STOP solution 10 ml (Ready to use) 4°C ARG81514-011 Plate sealer 4 strips Room temperature Summary Product Description ARG81514 Human KLK12 ELISA Kit is an Enzyme Immunoassay kit for the quantification of Human KLK12 in serum, plasma (heparin, EDTA) and cell culture supernatants. Tested Reactivity Hu Tested Application ELISA Specificity There is no detectable cross-reactivity with other relevant proteins. Target Name KLK12 Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm. Sensitivity 78 pg/ml Sample Type Serum, plasma (heparin, EDTA) and cell culture supernatants. Standard Range 156 - 10000 pg/ml Sample Volume 100 µl www.arigobio.com 1/2 Precision Intra-Assay CV: 5.8% Inter-Assay CV: 6.2% Alternate Names KLKL5; Kallikrein-12; EC 3.4.21.-; KLK-L5; Kallikrein-like protein 5 Application Instructions Assay Time ~ 5 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. -

Kallikreins Emerge As New Regulators of Viral Infections
Cellular and Molecular Life Sciences https://doi.org/10.1007/s00018-021-03922-7 Cellular andMolecular Life Sciences REVIEW Kallikreins emerge as new regulators of viral infections Georgios Pampalakis1 · Eleni Zingkou2 · Christos Panagiotidis1 · Georgia Sotiropoulou2 Received: 14 June 2021 / Revised: 23 July 2021 / Accepted: 12 August 2021 © The Author(s), under exclusive licence to Springer Nature Switzerland AG 2021 Abstract Kallikrein-related peptidases (KLKs) or kallikreins have been linked to diverse (patho) physiological processes, such as the epidermal desquamation and infammation, seminal clot liquefaction, neurodegeneration, and cancer. Recent mount- ing evidence suggests that KLKs also represent important regulators of viral infections. It is well-established that certain enveloped viruses, including infuenza and coronaviruses, require proteolytic processing of their hemagglutinin or spike proteins, respectively, to infect host cells. Similarly, the capsid protein of the non-enveloped papillomavirus L1 should be proteolytically cleaved for viral uncoating. Consequently, extracellular or membrane-bound proteases of the host cells are instrumental for viral infections and represent potential targets for drug development. Here, we summarize how extracellular proteolysis mediated by the kallikreins is implicated in the process of infuenza (and potentially coronavirus and papillo- mavirus) entry into host cells. Besides direct proteolytic activation of viruses, KLK5 and 12 promote viral entry indirectly through proteolytic cascade events, like the activation of thrombolytic enzymes that also can process hemagglutinin, while additional functions of KLKs in infection cannot be excluded. In the light of recent evidence, KLKs represent potential host targets for the development of new antivirals. Humanized animal models to validate their key functions in viral infections will be valuable. -

Human Tissue Kallikreins: Physiologic Roles and Applications in Cancer
Subject Review Human Tissue Kallikreins: Physiologic Roles and Applications in Cancer Carla A. Borgon˜ o, Iacovos P. Michael, and Eleftherios P. Diamandis Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, and Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada Abstract The human genome contains at least 553 protease genes and Tissue kallikreins are members of the S1 family (clan SA) counting (4). Protease action is always irreversible and can of trypsin-like serine proteases and are present in at involve either indiscriminant and non-specific degradation of least six mammalian orders. In humans, tissue kallikreins protein substrates, as in apoptosis, or highly specific proteolytic (hK) are encoded by 15 structurally similar, steroid processing or limited hydrolysis of selected target proteins, hormone–regulated genes (KLK ) that colocalize to resulting in a functional change, as in prohormone activation. chromosome 19q13.4, representing the largest cluster Proteases are classified according to three major criteria: of contiguous protease genes in the entire genome. hKs location of the scissile peptide bond within the substrate are widely expressed in diverse tissues and implicated (terminal or internal), catalytic mechanism, and evolutionary in a range of normal physiologic functions from the relationships, as revealed by structure. On the basis of the first regulation of blood pressure and electrolyte balance to criterion, proteases are broadly categorized as either exo- or tissue remodeling, prohormone processing, neural endopeptidases, respectively. According to the second criterion, plasticity, and skin desquamation. Several lines of endopeptidases are divided into the well-known cysteine, serine, evidence suggest that hKs may be involved in cascade threonine, aspartic, and metalloprotease subgroups. -

A Peptide-Based Approach to Evaluate the Adaptability of Influenza a Virus to Humans Based on Its Hemagglutinin Proteolytic Cleavage Site
RESEARCH ARTICLE A peptide-based approach to evaluate the adaptability of influenza A virus to humans based on its hemagglutinin proteolytic cleavage site Marco R. Straus1,2, Gary R. Whittaker1,2* 1 Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell University, Ithaca, a1111111111 New York, United States of America, 2 New York Center of Excellence for Influenza Research and Surveillance, University of Rochester Medical Center, Rochester, New York, United States of America a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 Abstract Cleavage activation of the hemagglutinin (HA) protein by host proteases is a crucial step in OPEN ACCESS the infection process of influenza A viruses (IAV). However, IAV exists in eighteen different HA subtypes in nature and their cleavage sites vary considerably. There is uncertainty Citation: Straus MR, Whittaker GR (2017) A peptide-based approach to evaluate the adaptability regarding which specific proteases activate a given HA in the human respiratory tract. of influenza A virus to humans based on its Understanding the relationship between different HA subtypes and human-specific prote- hemagglutinin proteolytic cleavage site. PLoS ONE ases will be valuable in assessing the pandemic potential of circulating viruses. Here we uti- 12(3): e0174827. https://doi.org/10.1371/journal. lized fluorogenic peptides mimicking the HA cleavage motif of representative IAV strains pone.0174827 causing disease in humans or of zoonotic/pandemic potential and tested them with a range Editor: Charles J. Russell, St. Jude Children's of proteases known to be present in the human respiratory tract. Our results show that pep- Research Hospital, UNITED STATES tides from the H1, H2 and H3 subtypes are cleaved efficiently by a wide range of proteases Received: January 12, 2017 including trypsin, matriptase, human airway tryptase (HAT), kallikrein-related peptidases 5 Accepted: March 15, 2017 (KLK5) and 12 (KLK12) and plasmin.