Consolidated Annual Report 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Developments in Prokinetic Therapy for Gastric Motility Disorders
REVIEW published: 24 August 2021 doi: 10.3389/fphar.2021.711500 New Developments in Prokinetic Therapy for Gastric Motility Disorders Michael Camilleri* and Jessica Atieh Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States Prokinetic agents amplify and coordinate the gastrointestinal muscular contractions to facilitate the transit of intra-luminal content. Following the institution of dietary recommendations, prokinetics are the first medications whose goal is to improve gastric emptying and relieve symptoms of gastroparesis. The recommended use of metoclopramide, the only currently approved medication for gastroparesis in the United States, is for a duration of less than 3 months, due to the risk of reversible or irreversible extrapyramidal tremors. Domperidone, a dopamine D2 receptor antagonist, is available for prescription through the FDA’s program for Expanded Access to Investigational Drugs. Macrolides are used off label and are associated with tachyphylaxis and variable duration of efficacy. Aprepitant relieves some symptoms of gastroparesis. There are newer agents in the pipeline targeting diverse gastric (fundic, antral and pyloric) motor functions, including novel serotonergic 5-HT4 agonists, dopaminergic D2/3 antagonists, neurokinin NK1 antagonists, and ghrelin agonist. Novel Edited by: targets with potential to improve gastric motor functions include the pylorus, macrophage/ Jan Tack, inflammatory function, oxidative -

London, 29 May 2009 Doc.Ref
European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 29 May 2009 Doc.Ref. EMEA/CHMP/282149/2009 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE SUMMARY OF POSITIVE OPINION* for VEDROP International Nonproprietary Name (INN): tocofersolan On 29 May 2009 the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending to grant a marketing authorisation under exceptional circumstances for the medicinal product Vedrop, 50 mg/ml, oral solution indicated in vitamin E deficiency due to digestive malabsorption in paediatric patients suffering from congenital chronic cholestasis or hereditary chronic cholestasis. The applicant for this medicinal product is Orphan Europe S.A.R.L. The active substance of Vedrop is tocofersolan, an alimentary tract and metabolism medicinal product (ATC code A11 HA08, other plain vitamin preparations). Tocofersolan, is vitamin E that has been made water-soluble by attaching it to polyethylene glycol 1000, and thereby can be absorbed from the gut in patients who have difficulty absorbing fats and vitamin E from the diet due congenital chronic or hereditary chronic cholestasis. The use of vitamin E preparations in this population helps to prevent neurological deterioration due to vitamin E deficiency. The benefits with Vedrop are improvement or stabilisation of neurological abnormalities due to vitamin E deficiency in patients with congenital chronic or hereditary chronic cholestasis. The most common side effect is diarrhoea. A pharmacovigilance plan for Vedrop, as for all medicinal products, will be implemented as part of the marketing authorisation. The approved indication is: “Vedrop is indicated in vitamin E deficiency due to digestive malabsorption in paediatric patients suffering from congenital chronic cholestasis or hereditary chronic cholestasis, from birth (in term newborns) to 16 or 18 years of age, depending on the region”. -

International Journal of Medicine and Pharmaceutical Research
Wayal Sunil Anil et al, IJMPR, 2019, 7(6): 180-183 CODEN (USA): IJCPNH | ISSN: 2321-2624 International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr R E S E A R C H A R T I C L E Formulation and In-vitro Evaluation of Alosetron Oral Thin Films Wayal Sunil Anil1, G.S. Valluri2, Gampa Vijay Kumar3 Department of Pharmacy, KGR Institute of Technology and Management, Rampally, Kesara, Medchal, Telangana, India. A B S T R A C T Alosetron, is a 5-HT3 antagonist used for the management of severe diarrhea-predominant irritable bowel syndrome. In present study oral thin films of Alosetron were developed to have a faster on set of action. The oralthin films were developed by using polymers Guar gum, Pullulan and PVP K30.Oral thin films were prepared by employing solvent casting method. Propylene glycol was selected as permeation enhancer and plasticizer. Drug excipient compatibility studies were carried out by using FTIR, and it was observed that there were no interactions. Formulations were prepared with the varying concentrations polymers ranging from F1-F9, and all the formulations were evaluated for various physical parameters Physical appearance, Weight variation, Thickness, Folding endurance, Tensile strength, Drug content, Moisture uptake, Moisture content and all the results were found to be were found to be within the pharmacopeial limits, in-vitro drug release studies by using USP dissolution Apparatus Type II. Among all the 9 formulations F4 formulation which contain Pullulan 10mg and shown 97.06% cumulative drug release within 30 min. -

Comprehensive Pgx Report for 1 / 31 Examples of Different Levels of Evidence for Pgx Snps
Comprehensive PGx report for PERSONAL DETAILS Advanced Diagnostics Laboratory LLC CLIA:31D2149403 Phone: Fax: PATIENT DOB Address: 1030 North Kings Highway Suite 304 Cherry Hill, NJ 08034 GENDER FEMALE Website: http://advanceddiagnosticslaboratory.com/ SPECIMEN TYPE Oral Fluid LABORATORY INFORMATION ORDERING PHYSICIAN ACCESSION NUMBER 100344 FACILITY COLLECTION DATE 08/10/2020 RECEIVED DATE 08/14/2020 REPORT GENERATED 09/08/2020 LABORATORY DIRECTOR Dr. Jeanine Chiaffarano Current Patient Medication Clonidine (Catapres, Kapvay) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, extensive CYP1A2-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Losartan (Cozaar) The personalized pharmacogenomics profile of this patient reveals extensive CYP2C9-mediated metabolism, extensive CYP3A4-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Diltiazem (Cardizem, Tiazac) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, intermediate CYP2C19-mediated metabolism, and extensive CYP3A5- mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Labetalol (Normodyne, Trandate) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, and intermediate CYP2C19-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Mycophenolate mofetil (Myfortic, CellCept) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, extensive CYP3A5-mediated metabolism, and extensive CYP2C8-mediated metabolism. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Annexes to the EMA Annual Report 2009
Annual report 2009 Annexes The main body of this annual report is available on the website of the European Medicines Agency (EMA) at: http://www.ema.europa.eu/htms/general/direct/ar.htm 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E-mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2010. Reproduction is authorised provided the source is acknowledged. Contents Annex 1 Members of the Management Board..................................................... 3 Annex 2 Members of the Committee for Medicinal Products for Human Use .......... 5 Annex 3 Members of the Committee for Medicinal Products for Veterinary Use ....... 8 Annex 4 Members of the Committee on Orphan Medicinal Products .................... 10 Annex 5 Members of the Committee on Herbal Medicinal Products ..................... 12 Annex 6 Members of the Paediatric Committee................................................ 14 Annex 7 National competent authority partners ............................................... 16 Annex 8 Budget summaries 2008–2009 ......................................................... 27 Annex 9 European Medicines Agency Establishment Plan .................................. 28 Annex 10 CHMP opinions in 2009 on medicinal products for human use .............. 29 Annex 11 CVMP opinions in 2009 on medicinal products for veterinary use.......... 53 Annex 12 COMP opinions in 2009 on designation of orphan medicinal products -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -
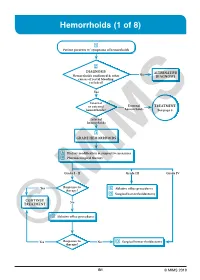
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine -

A Four-Country Comparison of Healthcare Systems, Implementation
Neurogastroenterology & Motility Neurogastroenterol Motil (2014) 26, 1368–1385 doi: 10.1111/nmo.12402 REVIEW ARTICLE A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders A report of the Rome Foundation Working Team on cross-cultural, multinational research M. SCHMULSON,* E. CORAZZIARI,† U. C. GHOSHAL,‡ S.-J. MYUNG,§ C. D. GERSON,¶ E. M. M. QUIGLEY,** K.-A. GWEE†† & A. D. SPERBER‡‡ *Laboratorio de Hıgado, Pancreas y Motilidad (HIPAM)-Department of Experimental Medicine, Faculty of Medicine-Universidad Nacional Autonoma de Mexico (UNAM). Hospital General de Mexico, Mexico City, Mexico †Gastroenterologia A, Department of Internal Medicine and Medical Specialties, University La Sapienza, Rome, Italy ‡Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow, India §Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea ¶Division of Gastroenterology, Mount Sinai School of Medicine, New York, NY, USA **Division of Gastroenterology and Hepatology, Houston Methodist Hospital and Weill Cornell Medical College, Houston, TX, USA ††Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore ‡‡Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel Key Messages • This report identified seven key issues related to healthcare provision that may impact how patients with FGIDs are investigated, diagnosed and managed. • Variations in healthcare provision around the world in patients with FGIDs have not been reviewed. • We compared four countries that are geographically and culturally diverse, and exhibit differences in the healthcare coverage provided to their population: Italy, South Korea, India and Mexico. • Since there is a paucity of publications relating to the issues covered in this report, some of the findings are based on the authors’ personal perspectives, press reports and other published sources. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole