Pelvic Inflammatory Disease (PID)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dyspareunia: an Integrated Approach to Assessment and Diagnosis
PROBLEMS IN FAMILY PRACTICE Dyspareunia: An Integrated Approach to Assessment and Diagnosis Genell Sandberg, PhD, and Randal P. Quevillon, PhD Seattle, Washington, and Vermillion, South Dakota Dyspareunia, or painful intercourse, is frequently referred to as the most common female sexual dysfunction. It can occur singly or be manifested in combination with other psychosexual disorders. Diagnosis of dyspareunia is appropriate in cases in which the experience of pain is persistent and severe. There has been little agreement concerning the origin of dyspareunia. Or ganic conditions and psychological variables have alternately been pre sented as major factors in causality. There is a presumed high incidence of physical disease associated with dyspareunia when compared with other female sexual dysfunctions. In the majority of cases, however, organic fac tors are thought to be rare in contrast with sexual issues and interpersonal or intrapsychic difficulties as a cause of continuing problems. The finding of an organic basis for dyspareunia does not rule out emotional or psychogenic causes. Thorough and extensive gynecologic and psycholog ical evaluation is essential in cases of dyspareunia. The etiology of dys pareunia should be viewed on a continuum from primarily physical to primar ily psychological with many women falling in the middle area. recurrent pattern of genital pain during or im Dyspareunia and vaginismus are undeniably linked, A mediately after coitus is the basis for the diagnosis and repeated dyspareunia is likely to result in vaginis of dyspareunia.1 The Diagnostic and Statistical Man mus, as vaginismus may be the causative factor in ual of Mental Disorders (DSM-III)2 has included dys dyspareunia.6-7 The difference between vaginismus pareunia under the classification of psychosexual dis and dyspareunia is that intromission is generally pain orders. -

Pelvic Inflammatory Disease (PID) PELVIC INFLAMMATORY DISEASE (PID)
Clinical Prevention Services Provincial STI Services 655 West 12th Avenue Vancouver, BC V5Z 4R4 Tel : 604.707.5600 Fax: 604.707.5604 www.bccdc.ca BCCDC Non-certified Practice Decision Support Tool Pelvic Inflammatory Disease (PID) PELVIC INFLAMMATORY DISEASE (PID) SCOPE RNs (including certified practice RNs) must refer to a physician (MD) or nurse practitioner (NP) for all clients who present with suspected PID as defined by pelvic tenderness and lower abdominal pain during the bimanual exam. ETIOLOGY Pelvic inflammatory disease (PID) is an infection of the upper genital tract that involves any combination of the uterus, endometrium, ovaries, fallopian tubes, pelvic peritoneum and adjacent tissues. PID consists of ascending infection from the lower-to-upper genital tract. Prompt diagnosis and treatment is essential to prevent long-term sequelae. Most cases of PID can be categorized as sexually transmitted and are associated with more than one organism or condition, including: Bacterial: Chlamydia trachomatis (CT) Neisseria gonorrhoeae (GC) Trichomonas vaginalis Mycoplasma genitalium bacterial vaginosis (BV)-related organisms (e.g., G. vaginalis) enteric bacteria (e.g., E. coli) (rare; more common in post-menopausal people) PID may be associated with no specific identifiable pathogen. EPIDEMIOLOGY PID is a significant public health problem. Up to 2/3 of cases go unrecognized, and under reporting is common. There are approximately 100,000 cases of symptomatic PID annually in Canada; however, PID is not a reportable infection so, exact -

3-Year Results of Transvaginal Cystocele Repair with Transobturator Four-Arm Mesh: a Prospective Study of 105 Patients
Arab Journal of Urology (2014) 12, 275–284 Arab Journal of Urology (Official Journal of the Arab Association of Urology) www.sciencedirect.com ORIGINAL ARTICLE 3-year results of transvaginal cystocele repair with transobturator four-arm mesh: A prospective study of 105 patients Moez Kdous *, Fethi Zhioua Department of Obstetrics and Gynecology, Aziza Othmana Hospital, Tunis, Tunisia Received 27 January 2014, Received in revised form 1 May 2014, Accepted 24 September 2014 Available online 11 November 2014 KEYWORDS Abstract Objectives: To evaluate the long-term efficacy and safety of transobtura- tor four-arm mesh for treating cystoceles. Genital prolapse; Patients and methods: In this prospective study, 105 patients had a cystocele cor- Cystocele; rected between January 2004 and December 2008. All patients had a symptomatic Transvaginal mesh; cystocele of stage P2 according to the Baden–Walker halfway stratification. We Polypropylene mesh used only the transobturator four-arm mesh kit (SurgimeshÒ, Aspide Medical, France). All surgical procedures were carried out by the same experienced surgeon. ABBREVIATIONS The patients’ characteristics and surgical variables were recorded prospectively. The VAS, visual analogue anatomical outcome, as measured by a physical examination and postoperative scale; stratification of prolapse, and functional outcome, as assessed by a questionnaire TOT, transobturator derived from the French equivalents of the Pelvic Floor Distress Inventory, Pelvic tape; Floor Impact Questionnaire and the Pelvic Organ Prolapse–Urinary Incontinence- TVT, tension-free Sexual Questionnaire, were considered as the primary outcome measures. Peri- vaginal tape; and postoperative complications constituted the secondary outcome measures. TAPF, tendinous arch Results: At 36 months after surgery the anatomical success rate (stage 0 or 1) was of the pelvic fascia; 93%. -

Dysmenorrhoea
[ Color index: Important | Notes| Extra | Video Case ] Editing file link Dysmenorrhoea Objectives: ➢ Define dysmenorrhea and distinguish primary from secondary dysmenorrhea ➢ • Describe the pathophysiology and identify the etiology ➢ • Discuss the steps in the evaluation and management options References : Hacker and moore, Kaplan 2018, 428 boklet ,433 , video case Done by: Omar Alqahtani Revised by: Khaled Al Jedia DYSMENORRHEA Definition: dysmenorrhea is a painful menstruation it could be primary or secondary Primary dysmenorrhea Definition: Primary dysmenorrhea refers to recurrent, crampy lower abdominal pain, along with nausea, vomiting, and diarrhea, that occurs during menstruation in the absence of pelvic pathology. It is the most common gynecologic complaint among adolescent girls. Characteristic: The onset of pain generally does not occur until ovulatory menstrual cycles are established. Maturation of the hypothalamic-pituitary-gonadal axis leading to ovulation occurs in half of the teenagers within 2 years post-menarche, and the majority of the remainder by 5 years post-menarche. (so mostly it’s occur 2-5 years after first menstrual period) • The symptoms typically begin several hours prior to the onset of menstruation and continue for 1 to 3 days. • The severity of the disorder can be categorized by a grading system based on the degree of menstrual pain, the presence of systemic symptoms, and impact on daily activities Pathophysiology Symptoms appear to be caused by excess production of endometrial prostaglandin F2α resulting from the spiral arteriolar constriction and necrosis that follow progesterone withdrawal as the corpus luteum involutes. The prostaglandins cause dysrhythmic uterine contractions, hypercontractility, and increased uterine muscle tone, leading to uterine ischemia. -

Vaginitis and Abnormal Vaginal Bleeding
UCSF Family Medicine Board Review 2013 Vaginitis and Abnormal • There are no relevant financial relationships with any commercial Vaginal Bleeding interests to disclose Michael Policar, MD, MPH Professor of Ob, Gyn, and Repro Sciences UCSF School of Medicine [email protected] Vulvovaginal Symptoms: CDC 2010: Trichomoniasis Differential Diagnosis Screening and Testing Category Condition • Screening indications – Infections Vaginal trichomoniasis (VT) HIV positive women: annually – Bacterial vaginosis (BV) Consider if “at risk”: new/multiple sex partners, history of STI, inconsistent condom use, sex work, IDU Vulvovaginal candidiasis (VVC) • Newer assays Skin Conditions Fungal vulvitis (candida, tinea) – Rapid antigen test: sensitivity, specificity vs. wet mount Contact dermatitis (irritant, allergic) – Aptima TMA T. vaginalis Analyte Specific Reagent (ASR) Vulvar dermatoses (LS, LP, LSC) • Other testing situations – Vulvar intraepithelial neoplasia (VIN) Suspect trich but NaCl slide neg culture or newer assays – Psychogenic Physiologic, psychogenic Pap with trich confirm if low risk • Consider retesting 3 months after treatment Trichomoniasis: Laboratory Tests CDC 2010: Vaginal Trichomoniasis Treatment Test Sensitivity Specificity Cost Comment Aptima TMA +4 (98%) +3 (98%) $$$ NAAT (like GC/Ct) • Recommended regimen Culture +3 (83%) +4 (100%) $$$ Not in most labs – Metronidazole 2 grams PO single dose Point of care – Tinidazole 2 grams PO single dose •Affirm VP III +3 +4 $$$ DNA probe • Alternative regimen (preferred for HIV infected -

Necrotizing Fasciitis Complicating Female Genital Mutilation: Case Report Abdalla A
EMHJ • Vol. 16 No. 5 • 2010 Eastern Mediterranean Health Journal La Revue de Santé de la Méditerranée orientale Case report Necrotizing fasciitis complicating female genital mutilation: case report Abdalla A. Mohammed 1 and Abdelazeim A. Mohammed 1 Introduction Case report On examination the she was very ill; she had a temperature of 40.2 ºC, pulse Necrotizing fasciitis is a deep-seated in- A 7-year-old girl presented to Kas- of 104 beats per minute and blood pres- fection of the subcutaneous tissue that sala New Hospital on 2 March 2005 sure of 90/60 mmHg. There was exten- results in the progressive destruction of with high fever following FGM. The sive perineal and anterior abdominal fascia and fat; it easily spreads across the procedure had been done 7 days prior wall necrosis (Figure 1). The left labium fascial plane within the subcutaneous to admission in a mass female genital majus, the lower three-quarters of the tissue [1]. It begins locally at the site of cutting in the village during the first left labium minus and most of the mons the trauma, which may be severe, minor week of the school summer vacation. pubis were eaten away. The clitoris was or even non-apparent. The affected After the cutting, a herbal powder was preserved. There was extensive loss of skin becomes very painful without any applied to the wound. No antibiotic skin and subcutaneous fat of the right grossly visible change. With progression was given. During that period she ex- inguinal region. Superficial skin ulcera- of the disease, tissues become swollen, perienced high fever and difficulty in tion reached the umbilicus. -

BD™ Gardnerella Selective Agar with 5% Human Blood
INSTRUCTIONS FOR USE – READY-TO-USE PLATED MEDIA PA-254094.06 Rev.: July 2014 BD Gardnerella Selective Agar with 5% Human Blood INTENDED USE BD Gardnerella Selective Agar with 5% Human Blood is a partially selective and differential medium for the isolation of Gardnerella vaginalis from clinical specimens. PRINCIPLES AND EXPLANATION OF THE PROCEDURE Microbiological method. Gardnerella vaginalis is considered to be one of the organisms causing vaginitis.1-4 Although the organism may be present in a high percentage of normal women in the vaginal flora, its importance as a cause of non-specific vaginitis (also called bacterial vaginosis) has never been questioned. In symptomatic women, G. vaginalis frequently is associated with anaerobes such as Prevotella bivia, P. disiens, Mobiluncus, Peptostreptococcus, and/or others which are a regular part of the urethral or intestinal, but not vaginal flora. In non-specific vaginitis, normal Lactobacillus flora is reduced or absent. Gardnerella vaginalis is considered to be the indicator organism for non-specific vaginitis which, in fact, is a polymicrobial infection.3,4 Although non- culture methods such as a direct Gram stain have been recommended in recent years for genital specimens, culture is still preferred by many laboratories.1,5 G. vaginalis may also be responsible for a variety of other diseases such as preterm birth, chorioamnionitis, urinary tract infections, newborn infections, and septicemia.6 The detection of the organism on routinely used media is difficult since Gardnerella and other -

Endometriosis-Associated Dyspareunia: the Impact on Women’S Lives Elaine Denny, Christopher H Mann
ARTICLE J Fam Plann Reprod Health Care: first published as 10.1783/147118907781004831 on 1 July 2007. Downloaded from Endometriosis-associated dyspareunia: the impact on women’s lives Elaine Denny, Christopher H Mann Abstract pain was found to limit sexual activity for the majority of the sample, with a minority ceasing to be sexually active. Background and methodology Endometriosis is a Lack of sexual activity resulted in a lowering of self- chronic condition in which endometrial glands and esteem and a negative effect on relationships with stroma are present outside of the uterus. Whereas partners, although the experience differed between chronic pelvic pain is the most commonly experienced younger and older women. pain of endometriosis, many women also suffer from deep dyspareunia. In order to determine how much of an Discussion and conclusions The experience of impact endometriosis-associated dyspareunia has on dyspareunia is a significant factor in the quality of life and the lives and relationships of women a qualitative study relationships for women living with endometriosis. For using semi-structured interviews, supplemented with most of the women in the study it was very severe and quantitative data on the extent of dyspareunia, was resulted in their reducing or curtailing sexual activity. conducted in a dedicated endometriosis clinic in the Qualitative research can produce salient data that West Midlands, UK with 30 women aged from 19 to 44 highlight the impact of dyspareunia on self-esteem and years. sexual relationships. Results The main outcome measures were the extent of Keywords dyspareunia, endometriosis, qualitative dyspareunia within the sample of women, and the impact research, quality of life, sexual relationships of dyspareunia on quality of life. -

Vaginitis No Disclosures Related to This Topic
Vaginitis No disclosures related to this topic Is the wet prep out of the building? Images are cited with permissions Barbara S. Apgar, MD, MS Professor of Family Medicine University of Michigan Health Center Michigan Medicine Ann Arbor, Michigan Women with vaginal discharge Is vaginal discharge ever “normal ”? Normal 30% Bacterial vaginosis 23-50% Few primary studies and most of low quality. Candida vaginitis 20-25% Quantity and quality of vaginal discharge varies considerably across women and during the Mixed 20% menstrual cycle. Desquamative inflammatory 8% Symptom of vaginal discharge is non-specific. Vaginitis Vaginal discharge is often thought to be vaginitis. Trichomoniasis 5-15% Vaginal symptoms are very common Patient with chronic vaginal discharge Presence or absence of a microbe corresponds poorly with the presence or absence of 17 year old GO complains of lots of heavy white symptoms. vaginal discharge which is bothersome. No agreement about timing, color or Regular periods, denies any sexual activity. characteristics of discharge among women with Numerous evaluations for STI’s, all negative. vaginal discharge Treated for vaginal candida, BV and trich Most women think vagina should be “dry ”. although there was no evidence for any Vaginal wetness may be normal . infection and did not resolve discharge. Schaaf et al. Arch Intern Med 1999;150. Physiologic vaginal discharge 17 year old Chronic vaginal Patients and providers may consider that a thick discharge white discharge is most frequently caused by candidiasis. Always wears a pad May lead to repeated use of unnecessary antifungal therapy and prompt concerns of Diagnosis? recurrent infection if not resolved. -

Painful Sex (Dyspareunia) Labia Majora a Guide for Women Labia Minora 1
Clitoris Painful Sex (Dyspareunia) Labia majora A Guide for Women Labia minora 1. What is dyspareunia? Perineum 2. How common is dyspareunia? 3. What causes dyspareunia? 4. How is dyspareunia diagnosed? 5. How is dyspareunia treated? These issues may start suddenly or occur gradually over a period of time and may be traced back to an event such as What is dysperunia? a previous infection. Additionally, certain disorders of the Dyspareunia, or female sexual pain, is a term used to describe urethra (the tube through which urine is emptied from the pelvic and/or vaginal pain during intercourse. The duration of bladder) can cause significant pain involving the vagina. Ex- the pain can be limited to the duration of intercourse but may last amples include urethritis (inflammation/pain in the urethra, for up to 24 hours after intercourse has finished. The duration sometimes caused by sexually transmitted diseases) and of symptoms varies widely and sometimes can be traced back urethral diverticulum (a weakness in the wall of the urethra to a specific time or event. It is often difficult to diagnose the which forms a pocket in which urine can be trapped). exact source of discomfort (muscular, vascular, foreign bodies, • Musculoskeletal issues. At times, women will describe a surgery, trauma, aging, emotional) as well as to implement the pain like being ‘stabbed in the vagina’ or chronic soreness. correct treatment options. This can be related to increased tension of the pelvic floor musculature so that it cannot properly relax. This phenom- How common is dyspareunia? enon (levator spasm) can cause constant or intermittent pain Dyspareunia is common but probably under-reported. -

Human Microbiota Network: Unveiling Potential Crosstalk Between the Different Microbiota Ecosystems and Their Role in Health and Disease
nutrients Review Human Microbiota Network: Unveiling Potential Crosstalk between the Different Microbiota Ecosystems and Their Role in Health and Disease Jose E. Martínez †, Augusto Vargas † , Tania Pérez-Sánchez , Ignacio J. Encío , Miriam Cabello-Olmo * and Miguel Barajas * Biochemistry Area, Department of Health Science, Public University of Navarre, 31008 Pamplona, Spain; [email protected] (J.E.M.); [email protected] (A.V.); [email protected] (T.P.-S.); [email protected] (I.J.E.) * Correspondence: [email protected] (M.C.-O.); [email protected] (M.B.) † These authors contributed equally to this work. Abstract: The human body is host to a large number of microorganisms which conform the human microbiota, that is known to play an important role in health and disease. Although most of the microorganisms that coexist with us are located in the gut, microbial cells present in other locations (like skin, respiratory tract, genitourinary tract, and the vaginal zone in women) also play a significant role regulating host health. The fact that there are different kinds of microbiota in different body areas does not mean they are independent. It is plausible that connection exist, and different studies have shown that the microbiota present in different zones of the human body has the capability of communicating through secondary metabolites. In this sense, dysbiosis in one body compartment Citation: Martínez, J.E.; Vargas, A.; may negatively affect distal areas and contribute to the development of diseases. Accordingly, it Pérez-Sánchez, T.; Encío, I.J.; could be hypothesized that the whole set of microbial cells that inhabit the human body form a Cabello-Olmo, M.; Barajas, M. -
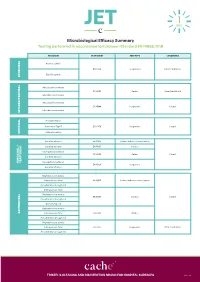
JET Microbiological Efficacy Summary
Microbiological Efficacy Summary Testing performed in accordance to European Standard EN 14885:2018 ORGANISM TEST NORM TEST TYPE CONDITIONS Bacillus subtilis EN 13704 Suspension Clean 1 and Dirty 1 Bacillus cereus SPORICIDAL Mycobacterium terrae EN 14563 Carrier Clean 1 and Dirty 2 Mycobacterium avium Mycobacterium terrae EN 14348 Suspension Clean 1 Mycobacterium avium MYCOBACTERICIDAL Poliovirus Type 1 Adenovirus Type 5 EN 14476 Suspension Clean 1 Murine Norovirus VIRUCIDAL Candida albicans EN 16615 Surface with mechanical action Candida albicans EN 13697 Surface Aspergillus brasiliensis EN 14562 Carrier Clean 1 Candida albicans YEASTICIDAL FUNGICIDAL / FUNGICIDAL Aspergillus brasiliensis EN 13624 Suspension Candida albicans Staphylococcus aureus Enterococcus hirae EN 16615 Surface with mechanical action Pseudomonas aeruginosa Enterococcus hirae Staphylococcus aureus EN 13697 Surface Clean 1 Pseudomonas aeruginosa Escherichia coli Staphylococcus aureus BACTERICIDAL Enterococcus hirae EN 14561 Carrier Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae EN 13727 Suspension Clean 1 and Dirty 1 Pseudomonas aeruginosa TRISTEL’S CLEANING AND DISINFECTION BRAND FOR HOSPITAL SURFACES Page 1 of 3 Additional Testing TEST METHOD RNA DNA / Polyacrylamide gel electrophoresis (PAGE) ORGANISM TEST METHOD TEST TYPE CONDITIONS Acanthamoeba castellanii cysts Following the method of EN 13704 Suspension Clean 1 PROTOZOA Bacillus subtilis EN 17126 Suspension Clean 1 Bacillus cereus Clostridium difficile EN 13704 Suspension Clean 1 and Dirty 1