Hypoxic Blackwater Event Severely Impacts Murray Crayfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 8. Aboriginal Water Values and Uses
Chapter 8. Aboriginal water values and uses Department of Environment, Land, Water and Planning 8. Aboriginal water values and uses The Murray-Darling Basin Plan requires Basin states to identify objectives and outcomes of water, based on Aboriginal values and uses of water, and have regard to the views of Traditional Owners on matters identified by the Basin Plan. Victoria engaged with Traditional Owner groups in the Water Resource Plan for the northern Victoria area to: • outline the purpose, scope and opportunity for providing water to meet Traditional Owner water objectives and outcomes through the Murray-Darling Basin Plan • define the role of the water resource plans in the Basin, including but not limited to the requirements of the Basin Plan (Chapter 10, Part 14) • provide the timeline for the development and accreditation of the Northern Victoria Water Resource Plan • determine each Traditional Owner group’s preferred means of engagement and involvement in the development of the Northern Victoria Water Resource Plan • continue to liaise and collaborate with Traditional Owner groups to integrate specific concerns and opportunities regarding the water planning and management framework. • identify Aboriginal water objectives for each Traditional Owner group, and desired outcomes The Water Resource Plan for the Northern Victoria water resource plan area, the Victorian Murray water resource plan area and the Goulburn-Murray water resource plan area is formally titled Victoria’s North and Murray Water Resource Plan for the purposes of accreditation. When engaging with Traditional Owners this plan has been referred to as the Northern Victoria Water Resource Plan and is so called in Chapter 8 of the Comprehensive Report. -

Nyah to Border Community Profile (Including Sunraysia, Victoria and NSW)
Nyah to Border community profile (including Sunraysia, Victoria and NSW) Irrigation region Key issues for the region 1. Region’s population — The population of the Nyah to Border region is approximately 60,000, including 3,500 farm businesses. 2. Gross value of irrigated agricultural production (GVIAP) • The drought affected gross value of irrigated agricultural production was an estimated $600 million for 2008-09i. The non drought gross value of irrigated agricultural production, based on the existing area, is estimated to be $800 millionii. 3. Water entitlements (approximate) • Surface Water Long-term Cap -700 GL. • Victorian high reliability water shares 481 GL NSW High Security - 190 GLiii , NSW General security — 75 GLiv • Groundwater entitlements - negligible. 4. Major enterprises –Major enterprises for the Nyah to Border region include wine grapes, citrus, table grapes, almonds, dried fruit, and vegetables. 5. Government buyback- The Commonwealth Government buyback cannot be separated out for the Nyah to Border region as it is part of greater Murray valley buyback program. 6. Water dependence — Very high capital investment on-farm and off-farm makes the regional economy highly water dependent. 7. Current status • Nyah to Border’s regional economy of around $3 billionv has a high dependence on irrigation, with wineries, packing sheds and other food processing reliant on a consistent supply of irrigated crops. Around 25% of employment is associated with irrigated horticulture and associated manufacturing. • There is a high population of 60,000 people including 3,500 growers, 65% of whom farm 26% of the irrigation area on small farms in community districts. These districts were established as Government irrigation schemes from 1887 to 1947. -

Sustainable Diversion Limit Adjustment
Sustainable Diversion Limit Adjustment Phase 2 Assessment Supply Measure Business Case: Nyah Floodplain Management Project i Supply Measure Business Case: Wallpolla Island Disclaimer This business case is one of nine Victorian environmental works projects. It was developed over two years ago and submitted for assessment in early 2015 by the Sustainable Diversion Limit Adjustment Assessment Committee (SDLAAC) in accordance with the inter-jurisdictional governance procedures that pertain to the Murray Darling Basin Plan. This business case relies on assumptions, estimates and other variables that were considered true, accurate and the best available information at the time of development. As a result of queries raised during the SDLAAC assessment process, there have been changes to certain elements of some projects, including engineering designs, methods of water supply and future operation. These details have not been incorporated or encapsulated in this or any of the other eight business cases relevant to the Sustainable Diversion Limit Adjustment Mechanism within the Murray Darling Basin Plan. There has, however, been no material changes to the environmental objectives and outcomes proposed to be achieved through these projects. All nine projects will be revisited for final development once Commonwealth funding is made available. The detailed cost estimates and other commercial-in-confidence information that originally formed part of this and the other eight business cases have been deliberately omitted from this version of the document. This is in recognition that this detail is no longer relevant given the time that has passed since these business cases were originally developed, new delivery methods are applicable in some cases and to ensure that value for money is achieved when these projects are issued for tender. -

Mallee Western
Holland Lake Silve r Ci Toupnein ty H Creek RA wy Lake Gol Gol Yelta C a l d e r H Pink Lake w y Merbein Moonlight Lake Ranfurly Mildura Lake Lake Walla Walla RA v A Lake Hawthorn n i k a e MILDURA D AIRPORT ! Kings Millewa o Irymple RA Billabong Wargan KOORLONG - SIMMONS TRACK Lake Channel Cullulleraine +$ Sturt Hwy SUNNYCLIFFS Meringur Cullulleraine - WOORLONG North Cardross Red Cliffs WETLANDS Lakes Karadoc Swamp Werrimull Sturt Hwy Morkalla RA Tarpaulin Bend RA Robinvale HATTAH - DUMOSA TRACK Nowingi Settlement M Rocket u Road RA r ra Lake RA y V a lle y H w HATTAH - RED y OCRE TRACK MURRAY SUNSET Lake - NOWINGI Bitterang Sunset RA LINE TRACK HATTAH - CALDER HIGHWAY EAST Lake Powell Raak Plain RA Lake Mournpall Chalka MURRAY SUNSET Creek RA - ROCKET LAKE TRACK WEST Lake Lockie WANDOWN - NORTH BOUNDARY MURRAY SUNSET Hattah - WILDERNESS PHEENYS TRACK MURRAY SUNSET - Millewa LAST HOPE TRACK MURRAY SUNSET South RA MURRAY SUNSET Kia RA - CALDER ANNUELLO - MURRAY SUNSET - - MENGLER ROAD HIGHWAY WEST NORTH WEST MURRAY SUNSET - +$ LAST HOPE TRACK NORTH EAST BOUNDARY LAST HOPE TRACK MURRAY SUNSET - SOUTH EAST SOUTH EAST LAST HOPE TRACK MURRAY SUNSET SOUTH EAST - TRINITA NORTH BOUNDARY +$ MURRAY SUNSET ANNUELLO - MENGLER MURRAY SUNSET - - EASTERN MURRAY SUNSET ROAD WEST TRINITA NORTH BOUNDARY - WILDERNESS BOUNDARY WEST Berrook RA Mount Crozier RA ANNUELLO - BROKEN GLASS TRACK WEST MURRAY SUNSET - SOUTH MERIDIAN ROAD ANNUELLO - SOUTH WEST C BOUNDARY ANNUELLO - a l d SOUTHERN e r BOUNDARY H w Berrook y MURRAY SUNSET - WYMLET BOUNDARY MURRAY SUNSET -

Annual Report 2019 - 2020
Sunraysia Rural Counselling Service Inc ANNUAL REPORT 2019 - 2020 Supported by the Australian and Victorian Governments ACKNOWLEDGMENTS The Sunraysia Rural Counselling Service Inc. wishes to acknowledge and sincerely thank the following organisations for their funding, contributions and support during the 2019-20 financial year: GRANTS Department of Agriculture • Australian Government funding for Murray-Darling Basin Economic Development Program - Small Business Project Department of Agriculture, Water and the Environment • Australian Government funding for the - Rural Financial Counselling Service Program - Small Business Financial Counselling Program Department of Jobs, Precincts and Regions • Victorian Government funding for the - Rural Financial Counselling Service Program - Small Business Financial Counselling Program - Employment of a Senior Rural Financial Counsellor Coordinator DONATIONS & CASH CONTRIBUTIONS NSW Department of Planning, Industry and Environment Murray Valley Winegrowers’ Inc Swan Hill Rural City Council . Y TASCO Petroleum H Wentworth Shire Council T - Cash contributions to SunRISE Mapping & Research A P IGA Community Chest and Victorian Lions Clubs M - Food vouchers to assist farmers in need E Mildura Millewa Country Women’s Association & - Fuel vouchers to assist Millewa farmers in need Y T ANZ Bank and Mildura Regional Development I - Funds raised from 2019 Sunraysia Agribusiness Conference to support primary producers R G E T PHOTOGRAPHIC IMAGES N Chris O’Connell Photography (Cover photo) I Gingerhouse Photography , T Philip Down S Darren Seiler U Andy Banks Photography R Imagine Pictures T : DESIGN O NewsAlert PR / Sunni Studios T T O PRINTING M Sunnyland Press R 2 U SUNRAYSIA RURAL COUNSELLING SERVICE 2 O 2019 - 2020 3 TABLE OF CONTENTS ACKNOWLEDGMENTS. 03 ABOUT US. 06 VISION, PHILOSOPHY, MOTTO. -

Environmental Watering in Victoria 2015-16
Victorian Environmental Water Holder Environmental watering in Victoria 2015-16 The Victorian Environmental Water Holder acknowledges the contribution of its partners, particularly waterway managers, in managing environmental water to improve the health of rivers, wetlands and floodplains. Acknowledgement of Country The Victorian Environmental Water Holder acknowledges Aboriginal Traditional Owners within Victoria, their rich culture and their spiritual connection to Country. The contribution and interests of Aboriginal people and organisations in the management of land and natural resources is also recognised and acknowledged. Aboriginal and Torres Strait Islander people are warned that this document may contain images or names of deceased persons. Foreword 2 Central region 32 Yarra system 34 Introduction 4 Tarago system 36 Why is environmental watering important? 4 Maribyrnong system 38 Carryover and trade 6 Werribee system 40 Water donations 6 Moorabool system 42 Partnerships 6 Lower Barwon wetlands 44 Funding research and river improvements 6 Case study: Less silt and more habitat Highlights of environmental watering in the Yarra River 46 in Victoria 7 Western region 50 Protecting waterbirds in climate change 10 Glenelg system 52 Wimmera system 54 People making a splash 14 Wimmera-Mallee wetlands 56 Donating water 14 Case study: Building a strong Wimmera River 60 From farmland to wetland 16 Northern region 64 Gippsland region 18 Goulburn system 66 Latrobe system 20 Broken system 68 Thomson River 22 Goulburn-Broken wetlands 70 Macalister River 24 Campaspe system 72 Snowy River 26 Central Murray wetlands 74 Case study: Environmental watering gives Lower Murray wetlands 76 threatened fish a brighter future 28 Loddon system 78 The Living Murray icon sites 80 Ovens system 88 Case study: Barmah wetlands alive with waterbirds 90 Case study: Rehabilitating Lake Elizabeth to its former glory 93 Glossary 96 Summary of environmental water delivery 2015–16 97 Emma Coats from the VEWH with Murray cod, by Rachel Wood. -

Mildura History & Heritage Trail
suggested itineraries mildura history & heritage trail Day 1 - Travel Distance – 195km Time required approx. 6 - 7 hours Day 2 - Travel Distance – 32km Time required approx. 5 – 6 hours. The Mildura History and Heritage Trail has been designed to give you a sample of the region’s unique history and is split over two days, showcasing indigenous history, sights from the early irrigation settlement of the region, wartime history and post war soldier settlement. This itinerary, when downloaded to your smart device, is fully interactive, so you can choose to follow the itinerary as offered, or pick just those items that peak your interest, after all this is all about you! Simply select the destination and hit ‘Take Me There’ and your smart device will bring up the destination on Google Maps. Just select start and Google Maps will direct you there – simple! Have a fantastic day, it’s time to step back in time and experience the journey that is truly Mildura. If you need any assistance along the way please give us a call on 1800 039 043. DAY ONE 2 RAAF Memorial and Museum (9.6km from previous point) During World War Two, Mildura Airport was home to the RAAF 1 Mildura Visitor Information & Booking Centre 2 Operational Training Unit (2OTU) and was responsible for Make the Mildura Visitor Information and Booking Centre the providing operational fighter conversion and training to pilots start of your journey of discovery of the Mildura region. Our before they were posted to their squadrons. Around 1247 pilots team of expert staff can help you plan your time in Mildura and were successfully trained at 2OTU and sadly 52 Aircrew and 7 share the secrets only a local would know. -

SCHEDULE to the HERITAGE OVERLAY the Requirements of This
MILDURA PLANNING SCHEME 14/03/2013 SCHEDULE TO THE HERITAGE OVERLAY VC85 The requirements of this overlay apply to both the heritage place and its associated land. PS Heritage Place External Internal Tree Outbuildings Included on Prohibited Name of Aboriginal Map Paint Alteration Controls or fences the Victorian uses may Incorporated heritage Ref Controls Controls Apply? which are not Heritage be Plan under place? Apply? Apply? exempt under Register permitted? Clause 43.01-2 Clause 43.01-3 under the Heritage Act 1995? Victorian Heritage Register HO104 Big Lizzie - - - - YesRefNo No No H1919 CA 4, Section 8, Calder Highway and Jamieson Avenue, Red Cliffs HO182 Kow Plains Homestead - - - - Yes Ref No No 99 Cowangie Road, South Cowangie No H688 HO6 Rio Vista - - - - Yes Ref No No 199-205 Currton Avenue, Mildura No H729 HO16 Former Methodist Church - - - - Yes Ref No No Corner Deakin Avenue and Tenth Street, No H588 Mildura HO184 Former Murrayville Consolidated School - - - - Yes Ref No No Francis Street and 33 Poole Street, Murrayville No H1185 HO126 Irymple Railway Station - - - - Yes Ref No No Railway Reserve Sec 42 Blk F Irymple No H1568 Avenue, Irymple HO148 Lock Nine Pumping Station - - - - Yes Ref No No Lock Nine Drive, Cullulleraine No H549 HO183 Murrayville Railway Station - - - - Yes Ref No No McKenzie Street Murrayville No H1580 HERITAGE OVERLAY – SCHEDULE PAGE 1 OF 13 MILDURA PLANNING SCHEME PS Heritage Place External Internal Tree Outbuildings Included on Prohibited Name of Aboriginal Map Paint Alteration Controls or fences the -

Mildura Social Indicators 2012
Document Control Job ID: 16627 BNE Job Name: MILDURA RCC Social Indicators Report 2012 Client: Mildura Rural City Council Client Contact: Renée Ficarra Project Manager: Sara Hoenig Email: [email protected] Telephone: 07 3831 0577 Document Name: AECgroup Report - Mildura Social Indicators Report 2012 Final Report.docx Last Saved: 27/8/2014 10:33 AM Version Date Reviewed Approved Draft Report 29/05/2014 SH ARP Final Report 29/07/2014 SH ARP Disclaimer: Whilst all care and diligence have been exercised in the preparation of this report, AEC Group Limited does not warrant the accuracy of the information contained within and accepts no liability for any loss or damage that may be suffered as a result of reliance on this information, whether or not there has been any error, omission or negligence on the part of AEC Group Limited or their employees. Any forecasts or projections used in the analysis can be affected by a number of unforeseen variables, and as such no warranty is given that a particular set of results will in fact be achieved. i Acknowledgements and Notes Acknowledgements The authors are grateful to Consultative Council on Obstetric & Paediatric Mortality & Morbidity (CCOPMM) and the Department of Health staff who provided advice and prepared the VPDC data for this report. The views and conclusions are those of the authors and do not necessarily represent those of CCOPMM or the Department of Health. Research Notes Following the release of the 2011 Census, the Mildura Social Indicators Report has been updated to include the most recent and relevant data available. -
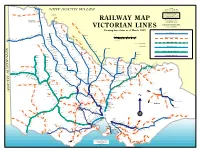
Railway Map Victorian Lines
Yelta Merbein West NOTES Mildura NEW SOUTH WALES All stations are shown with MerbeinIrymple their most recent known names. Redcliffs Abbreviations used Robinvale to Koorakee Morkalla Werrimull Karawinna Yatpool built by VR construction Meringurarrara BG = Broad Gauge (5' 3") Y Pirlta Thurla branch but never handed Benetook over to VR for traffic. Karween Merrinee SG = Standard Gauge (4' 8 1/2") Bambill Carwarp NG = Narrow Gauge (2' 6") Koorakee Boonoonar Benanee RAILWAY MAP Nowingi towards Millewa South Euston All lines shown are or were built by VR construction branch never handed over to VR for traffic, Nowingi Broad Gauge (5' 3") ownership sold to Brunswick Robinvale Plaster Mills 1942 unless otherwise shown. Balranald Bannerton Yangalake No attempt has been made to identify Yungara private railways or tourist lines being Hattah Margooya Impimi Koorkab VICTORIAN LINES run on closed VR lines Annuello Moolpa Kooloonong Trinita Koimbo Perekerten Showing line status as of March 1999 Natya Bolton Kiamal Coonimur Open BG track Kulwin Manangatang Berambong Tiega Piangil Stony Crossing Ouyen MILES Galah Leitpar Moulamein Cocamba Miralie Tueloga Walpeup Nunga 10 5 0 10 20 30 40 Mittyack Dilpurra Linga Underbool Torrita Chinkapook Nyah West Closed or out of use track Boinka Bronzewing Dhuragoon utye 0 5 10 20 30 40 50 60 T Pier Millan Coobool Panitya Chillingollah Pinnaroo Carina Murrayville Cowangie Pira Niemur KILOMETRES Gypsum Woorinen Danyo Nandaly Wetuppa I BG and 1 SG track Swan Hill Jimiringle Tempy Waitchie Wodonga open station Nyarrin Nacurrie Patchewollock Burraboi Speed Gowanford Pental Ninda Ballbank Cudgewa closed station Willa Turriff Ultima Lake Boga Wakool 2 BG and 1 SG track Yarto Sea Lake Tresco Murrabit Gama Deniliquin Boigbeat Mystic Park Yallakool Dattuck Meatian Myall Lascelles Track converted from BG to SG Berriwillock Lake Charm Caldwell Southdown Westby Koondrook Oaklands Burroin Lalbert Hill Plain Woomelang Teal Pt. -

Murray-Darling Basin Environmental Water Knowledge and Research Project Synthesis Report
Murray-Darling Basin Environmental Water Knowledge and Research Project Synthesis Report Nikki Thurgate, Julia Mynott, Lyn Smith and Nick Bond 9 201 Final Report CFE Publication 230 August Murray-Darling Basin Environmental Water Knowledge and Research Project Research Site Report Report prepared for the Department of the Environment and Energy, Commonwealth Environmental Water Office by La Trobe University, Centre for Freshwater Ecosystems (formerly Murray-Darling Freshwater Research Centre). Department of the Environment and Energy, Commonwealth Environmental Water Office GPO Box 787, Canberra, ACT, 2601 For further information contact: Nick Bond Nikki Thurgate Project Leader Project Co-ordinator Centre for Freshwater Ecosystems (formerly Murray–Darling Freshwater Research Centre) PO Box 821 Wodonga VIC 3689 Ph: (02) 6024 9640 (02) 6024 9647 Email: [email protected] [email protected] Web: https://www.latrobe.edu.au/freshwater-ecosystems/research/projects/ewkr Enquiries: [email protected] Report Citation: Thurgate NY, Mynott J, Smith L and Bond NR (2019) Murray-Darling Basin Environmental Water Knowledge and Research Project — Synthesis Report. Report prepared for the Department of the Environment and Energy, Commonwealth Environmental Water Office by La Trobe University, Centre for Freshwater Ecosystems, CFE Publication 230 August 2019 41p. Cover Image: Floodplain inundation Photographer: Centre for Freshwater Ecosystems Traditional Owner acknowledgement: La Trobe University Albury-Wodonga and Mildura campuses are located on the land of the Latje and Wiradjuri peoples. The Research Centre undertakes work throughout the Murray Darling Basin and acknowledge the traditional owners of this land and water. We pay respect to Elders past, present and future. Acknowledgements: We acknowledge the hard work of all EWKR project team members including all researchers, technicians and administrative staff whose work made the project a success and whose work this is. -

4 – Murray River & Floodplain
4 – Murray River & Floodplain - Robinvale to Merbein Regional Catchment Strategy Implementation Plan Mallee Regional Catchment Strategy 2013-19 This plan is a living document, which may be updated as required in light of new information or changing conditions. Whenever this document is revised, it will be uploaded to the Mallee CMA Website, replacing the old version. To allow for ongoing review and renewal processes, some sections of the document may not be entirely complete at the time of publishing. Publications produced by the Mallee Catchment Management Authority may be of assistance to you but the Mallee Catchment Management Authority and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purpose and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in any Mallee Catchment Management Authority publication. Version Control Version Number Prepared by Reviewed by Date 1 (Presented on Sean Dwyer Jo Latta 06/11/2013 internal blog) 2 Amy Leamon Jo Latta 17/03/2017 © Mallee Catchment Management Authority 2017 2 Contents Section 1 - Overview ............................................................................................................................... 5 Catchment Asset Significance ............................................................................................................ 6 Catchment Asset Value ......................................................................................................................