Phytophthora Ramorum and Pathogen Sporulation Potential
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
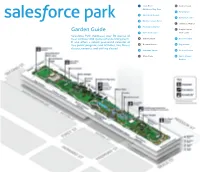
Salesforce Park Garden Guide
Start Here! D Central Lawn Children’s Play Area Garden Guide6 Palm Garden 1 Australian Garden Start Here! D Central Lawn Salesforce Park showcases7 California over Garden 50 species of Children’s Play Area 2 Mediterraneantrees and Basin over 230 species of understory plants. 6 Palm Garden -ã ¼ÜÊ ÊăØÜ ØÊèÜãE úØƀØÊèÃJapanese Maples ¼ÃØ Ê¢ 1 Australian Garden 3 Prehistoric¢ØÕ輫ÕØÊ£ØÂÜÃã«ó«ã«Üŧ¼«¹ĆãÃÜÜ Garden 7 California Garden ¼ÜÜÜŧÊÃØãÜŧÃØ¢ã«Ã£¼ÜÜÜũF Amphitheater Garden Guide 2 Mediterranean Basin 4 Wetland Garden Main Lawn E Japanese Maples Salesforce Park showcases over 50 species of 3 Prehistoric Garden trees and over 230 species of understory plants. A Oak Meadow 8 Desert Garden F Amphitheater It also offers a robust year-round calendar of 4 Wetland Garden Main Lawn free public programs and activities, like fitness B Bamboo Grove 9 Fog Garden Desert Garden classes, concerts, and crafting classes! A Oak Meadow 8 5 Redwood Forest 10 Chilean Garden B Bamboo Grove 9 Fog Garden C Main Plaza 11 South African 10 Chilean Garden Garden 5 Redwood Forest C Main Plaza 11 South African Garden 1 Children’s Australian Play Area Garden ABOUT THE GARDENS The botanist aboard the Endeavor, Sir Joseph Banks, is credited with introducing many plants from Australia to the western world, and many This 5.4 acre park has a layered soil system that plants today bear his name. balances seismic shifting, collects and filters storm- water, and irrigates the gardens. Additionally, the soil Native to eastern Australia, Grass Trees may grow build-up and dense planting help offset the urban only 3 feet in 100 years, and mature plants can be heat island effect by lowering the air temperature. -

A4 Template with Cover and Following Page
Powerful Owl Project Update – December 2015 Caroline Wilson, Holly Parsons & Janelle Thomas Thank-you to all of you for being involved with another successful year of the Powerful Owl Project. We had some changes this year, with our main grant funding finishing Caroline Wilson and Janelle Thomas from the Threatened Bird Network (TBN) and Holly Parsons from the Birds in Backyards Program took over the running of the project for BirdLife Australia. Generous donations from the NSW Twitch-a-thon has allowed us to complete this year’s research and allows us to continue in 2016. This season we have had over 120 registered volunteers involved, including over 50 new volunteers who were recruited to the project early this year. You have helped us monitor over 80 Powerful Owl breeding sites, allowing the project to cover a lot of ground throughout Greater Sydney, the NSW Central Coast and Newcastle. The data you have collected is really important for the effective management of urban Powerful Owl populations and this information is shared with land mangers and local councils. So thank-you! We really appreciate the amazing work carried out by our volunteers; with your help we have learned so much about these birds, and this information is helping us protect this unique and amazing species. Read on to hear about all we have achieved in 2015. Powerful Owl chick from 2015, peeking out of the hollow (taken by Christine Melrose) March 2015 workshop The March workshop was held to train our new volunteers, update everyone on the findings from the project and to say thank-you to our existing volunteers – some of which have been with us since 2011. -

Programa Fondecyt Informe Final Etapa 2015 Comisión Nacional De Investigacion Científica Y Tecnológica Version Oficial Nº 2
PROGRAMA FONDECYT INFORME FINAL ETAPA 2015 COMISIÓN NACIONAL DE INVESTIGACION CIENTÍFICA Y TECNOLÓGICA VERSION OFICIAL Nº 2 FECHA: 24/12/2015 Nº PROYECTO : 3130417 DURACIÓN : 3 años AÑO ETAPA : 2015 TÍTULO PROYECTO : EVOLUTIONARY AND DEVELOPMENTAL HISTORY OF THE DIVERSITY OF FLORAL CHARACTERS WITHIN OXALIDALES DISCIPLINA PRINCIPAL : BOTANICA GRUPO DE ESTUDIO : BIOLOGIA 1 INVESTIGADOR(A) RESPONSABLE : KESTER JOHN BULL HEREÑU DIRECCIÓN : COMUNA : CIUDAD : REGIÓN : METROPOLITANA FONDO NACIONAL DE DESARROLLO CIENTIFICO Y TECNOLOGICO (FONDECYT) Moneda 1375, Santiago de Chile - casilla 297-V, Santiago 21 Telefono: 2435 4350 FAX 2365 4435 Email: [email protected] INFORME FINAL PROYECTO FONDECYT POSTDOCTORADO OBJETIVOS Cumplimiento de los Objetivos planteados en la etapa final, o pendientes de cumplir. Recuerde que en esta sección debe referirse a objetivos desarrollados, NO listar actividades desarrolladas. Nº OBJETIVOS CUMPLIMIENTO FUNDAMENTO 1 1. Creating a database of morphological TOTAL La base de datos ya se encuentra en el sistema characters of perianth and androecium in the 52 PROTEUS y cuenta con el 733 registros genera of the Oxalidales from data gained from correspondientes a información acerca de 24 literature revision and direct observation of living variables morfológicas para 56 taxa de los collection and herbaria. Traits to be considered Oxalidales representando las siete familias y 51 are: presence or absence of calix and corolla, géneros del orden. aestivation pattern of calix and corolla, number of stamina, number of androecial cycles, relative position of stamina cycles (alternate-opposite), direction of stamen initiation, kind of stamina proliferation (primary or secondary). 2 2. Reconstructing the character state evolution of TOTAL Se ha hecho el estudio de reconstrucción de the abovementioned attributes using the available estados de carácter en base a parsimonia con phylogenetic data. -

Evergreen Trees Agonis Flexuosa
Evergreen Trees Agonis flexuosa – Peppermint Willow Graceful willow-like evergreen tree (but without the willows voracious root system) with reddish-brown, deeply furrowed bark to 25’-30’. New leaves and twigs have an attractive reddish cast; clustered small white flowers and brownish fruits are not particularly ornamental. Casaurina stricta – Beefwood Pendulous gray branches; resembles a pine somewhat; tolerates drought, heat, wind, fog. Growth to 20’- 30’. Cinnamomum camphora - Camphor Evergreen trees to 40 feet, with 20-foot spread.. In winter foliage is a shiny yellow green. In early spring new foliage may be pink, red or bronze, depending on tree. Unusually strong structure. Clusters of tiny, fragrant yellow flowers in profusion in May. Geijera parviflora- Australian Willow Evergreen trees with graceful, fine-textured leaves, to 30 feet, 20 feet wide. Main branches weep up and out; little branches hang down. Much of the grace of a willow, much of the toughness of eucalyptus, moderate growth and deep non-invasive roots. Laurus nobilis – Grecian Laurel Slow growth 12-40’. Natural habit is compact, broad-based, often multi-stemmed, gradually tapering cone. Leaves lethery, aromatic. Clusters of small yellow flowers followed by black or purple berries. Magnolia Grandiflora – ‘Little Gem’- Dwarf Southern Magnolia Small tree to 20’ in height. Showy white flowers in the summer. Green glossy leaves. Maytenous boaria - Mayten Evergreen tree with slow to moderate growth to an eventual 30-50 feet, with a 15-foot spread, with long and pendulous branchlets hanging down from branches, giving tree a graceful look. Habit and leaves somewhat like a small scale weeping willow. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

The Wood Cross Sections of Hermann Nördlinger (1818–1897)
IAWA Journal, Vol. 29 (4), 2008: 439–457 THE WOOD CROSS SECTIONS OF HERMANN NÖRDLINGER (1818–1897) Ben Bubner Leibniz-Zentrum für Agrarlandschaftsforschung (ZALF) e.V., Institut für Landschaftsstoffdynamik, Eberswalder Str. 84, 15374 Müncheberg, Germany [E-mail: [email protected]] SUMMARY Hermann Nördlinger (1818–1897), forestry professor in Hohenheim, Germany, published a series of wood cross sections in the years 1852 to 1888 that are introduced here to the modern wood anatomist. The sec- tions, which vary from 50 to 100 μm in thickness, are mounted on sheets of paper and their quality is high enough to observe microscopic details. Their technical perfection is as remarkable as the mode of distribution: sections of 100 wood species were presented in a box together with a booklet containing wood anatomical descriptions. These boxes were dis- tributed as books by the publisher Cotta, from Stuttgart, Germany, with a maximum circulation of 500 per volume. Eleven volumes comprise 1100 wood species from all over the world. These include not only conifers and broadleaved trees but also shrubs, ferns and palms representing a wide variety of woody structures. Excerpts of this collection were also pub- lished in Russian, English and French. Today, volumes of Nördlingerʼs cross sections are found in libraries throughout Europe and the United States. Thus, they are relatively easily accessible to wood anatomists who are interested in historic wood sections. A checklist with the content of each volume is appended. Key words: Cross section, wood collection, wood anatomy, history. INTRODUCTION Wood scientists who want to distinguish wood species anatomically rely on thin sec- tions mounted on glass slides and descriptions in books that are illustrated with micro- photographs. -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

Gum Trees Talk Notes
Australian Plants Society NORTH SHORE GROUP Eucalyptus, Angophora, Corymbia FAMILY MYRTACEAE GUM TREES OF THE KU-RING-GAI WILDFLOWER GARDEN Did you know that: • The fossil evidence for the first known Gum Tree was from the Tertiary 35-40 million years ago. • Myrtaceae is a very large family of over 140 genera and 3000 species of evergreen trees and shrubs. • There are over 900 species of Gum Trees in the Family Myrtaceae in Australia. • In the KWG, the Gum Trees are represented in the 3 genera: Eucalyptus, Angophora & Corymbia. • The name Eucalyptus is derived from the Greek eu = well and kalyptos = covered. BRIEF HISTORY E. obliqua The 18th &19th centuries were periods of extensive land exploration in Australia. Enormous numbers of specimens of native flora were collected and ended up in England. The first recorded scientific collection of Australian flora was made by Joseph Banks and Daniel Solander, during Sir James Cook’s 1st voyage to Botany Bay in April 1770. From 1800-1810, George Caley collected widely in N.S.W with exceptional skill and knowledge in his observations, superb preservation of plant specimens, extensive records and fluent expression in written records. It is a great pity that his findings were not published and he didn’t receive the recognition he deserved. The identification and classification of the Australian genus Eucalyptus began in 1788 when the French botanist Charles L’Heritier de Brutelle named a specimen in the British Museum London, Eucalyptus obliqua. This specimen was collected by botanist David Nelson on Captain Cook’s ill- fated third expedition in 1777 to Adventure Bay on Tasmania’s Bruny Is. -

Correa Study Group ISSN 1039-6926 ABN 56 654 053 676 Leader: Cherree Densley 9 Koroit-Port Fairy Road, Killarney, Vic, 3283 [email protected] Ph 03 5568 7226
ANPSA Correa Study Group ISSN 1039-6926 ABN 56 654 053 676 Leader: Cherree Densley 9 Koroit-Port Fairy Road, Killarney, Vic, 3283 [email protected] Ph 03 5568 7226 Admin & Editor: Russell Dahms 13 Everest Avenue, Athelstone, S.A. 5076 [email protected] Ph. 08 8336 5275 Membership fees: normal $10.00 Newsletter No.47 December 2012 electronic $6.00 EDITOR ’S COMMENTS This spring has brought with it very tough conditions here in South Australia with virtually Hello everyone, no rain for over two months now. I would like to introduce myself as the new One of the reasons for joining the correa group newsletter officer, membership officer and was that as a grower for the APS SA plant treasurer for the Correa Study Group. sales I have recently expanded the range of This has been my first year as a member of the correas I propagate – all from cuttings. ANPSA Correa Study Group. I have adopted I now have 20-30 species of correa and many the roles of membership officer, treasurer and of them are planted in my garden which is in newsletter editor while Cherree Densley the foothills east of Adelaide. The soil is remains the study group leader. predominantly clay with some topsoil added. My first main interaction with the study group Due to the lack of rain I have been giving any was the correa crawl which was held at Mt. of the correas that look like they are struggling Gambier this year. through their first summer additional deep watering. This was a wonderful opportunity to meet fellow study group members as well as experience firsthand many correa species new to me in Contents their natural habitat. -

Report by John Knight Blessed by a Gorgeous Autumn Day, and the Promise of an Interesting Program, a Large Gathering Arrived at Anne and Michael’S Durras Property
Report by John Knight Blessed by a gorgeous autumn day, and the promise of an interesting program, a large gathering arrived at Anne and Michael’s Durras property. The warm and dry conditions of recent weeks continue, and the expected display of Sunshine Wattle, Acacia terminalis did not eventuate, but the bushland around North Durras did present us with quite a diversity of plants to keep everyone interested. During a relaxed morning tea, enjoyed on the deck in the sunshine, the meeting kicked off with a ‘show and tell’ session. Phil and Catriona were once again to the fore, with a range of Banksias and Hakeas. A highlight was Hakea bakeriana, a medium sized shrub which is found in the heaths of the central coast. Foliage is a bright fresh green, and although it looks prickly, is quite benign. Brilliant pink to red flowers are borne on older wood, but still well presented. Catriona put in a plug for the Isopogon and Petrophile Study Group, which she and Phil lead. And with flowers as shown on her Isopogon cuneatus specimen, it is little wonder members get enthusiastic about these plants. Then comes the revelation that for success, they are best grafted onto hardy eastern states rootstock. Phil continues to experiment with this process, and is having good success using a hybrid of Isopogon mnoraifolius from the northern tablelands. Banksia vincentia, the most recently named Banksia, occurs in a swampy area near industrial development at Vincentia on the NSW South Coast. There are just a few plants remaining in the wild, and the population is seriously threatened by development and changed drainage patterns. -

Flora Survey on Hiltaba Station and Gawler Ranges National Park
Flora Survey on Hiltaba Station and Gawler Ranges National Park Hiltaba Pastoral Lease and Gawler Ranges National Park, South Australia Survey conducted: 12 to 22 Nov 2012 Report submitted: 22 May 2013 P.J. Lang, J. Kellermann, G.H. Bell & H.B. Cross with contributions from C.J. Brodie, H.P. Vonow & M. Waycott SA Department of Environment, Water and Natural Resources Vascular plants, macrofungi, lichens, and bryophytes Bush Blitz – Flora Survey on Hiltaba Station and Gawler Ranges NP, November 2012 Report submitted to Bush Blitz, Australian Biological Resources Study: 22 May 2013. Published online on http://data.environment.sa.gov.au/: 25 Nov. 2016. ISBN 978-1-922027-49-8 (pdf) © Department of Environment, Water and Natural Resouces, South Australia, 2013. With the exception of the Piping Shrike emblem, images, and other material or devices protected by a trademark and subject to review by the Government of South Australia at all times, this report is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. All other rights are reserved. This report should be cited as: Lang, P.J.1, Kellermann, J.1, 2, Bell, G.H.1 & Cross, H.B.1, 2, 3 (2013). Flora survey on Hiltaba Station and Gawler Ranges National Park: vascular plants, macrofungi, lichens, and bryophytes. Report for Bush Blitz, Australian Biological Resources Study, Canberra. (Department of Environment, Water and Natural Resources, South Australia: Adelaide). Authors’ addresses: 1State Herbarium of South Australia, Department of Environment, Water and Natural Resources (DEWNR), GPO Box 1047, Adelaide, SA 5001, Australia. -

Tasmania - from the Wet West to the Dry East
This collection is maintained with the assistance of the Tasmania - from the wet west to the dry east. Regional Branch of the Australian Plant Society. Influences on the development of the Tasmanian plant mix Montane moorland and cool oceanic heathland When Gondwana existed as a super Geology of Tasmania Vegetation Map of Tasmania The Tasmanian highland vegetation developed in isolation from the Australian Alps. Even during ice continent, Australia and Tasmania, Africa, ages, hundreds of kilometres of lowland vegetation separated the two high altitude environments. South America, New Zealand and Antarctica shared many plant families and some Montane plants have to cope with wide temperature fluctuations, with periods of below 0°C and Genera. exposure to winds. Cold may be prolonged if the ground freezes. Plants may be blanketed by snow or BASS STRAIT the mountains by cloud. Snowmelt or clear weather can cause intense rays of light, resulting in high For example, the protea family has members temperature. Wind or sun can dry the plant and soil. in all those land masses except Antarctica. The Southern Africa panel covers the protea family more fully. Plants require moisture and warmth. Small hard leaves offer Tasmania was the last land mass to break protection from the drying away from Antarctica. The opening of the effects of sun and wind. Low gap between these land masses allowed the ocean to circulate growth avoids wind. Branches around Antarctica, cooling the earth’s climate and so locking up grow close together to shelter vast quantities of water as ice. the parts of each plant.