BMC Evolutionary Biology Biomed Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download This Publication (PDF File)
PUBLIC LIBRARY of SCIENCE | plosgenetics.org | ISSN 1553-7390 | Volume 2 | Issue 12 | DECEMBER 2006 GENETICS PUBLIC LIBRARY of SCIENCE www.plosgenetics.org Volume 2 | Issue 12 | DECEMBER 2006 Interview Review Knight in Common Armor: 1949 Unraveling the Genetics 1956 An Interview with Sir John Sulston e225 of Human Obesity e188 Jane Gitschier David M. Mutch, Karine Clément Research Articles Natural Variants of AtHKT1 1964 The Complete Genome 2039 Enhance Na+ Accumulation e210 Sequence and Comparative e206 in Two Wild Populations of Genome Analysis of the High Arabidopsis Pathogenicity Yersinia Ana Rus, Ivan Baxter, enterocolitica Strain 8081 Balasubramaniam Muthukumar, Nicholas R. Thomson, Sarah Jeff Gustin, Brett Lahner, Elena Howard, Brendan W. Wren, Yakubova, David E. Salt Matthew T. G. Holden, Lisa Crossman, Gregory L. Challis, About the Cover Drosophila SPF45: A Bifunctional 1974 Carol Churcher, Karen The jigsaw image of representatives Protein with Roles in Both e178 Mungall, Karen Brooks, Tracey of various lines of eukaryote evolution Splicing and DNA Repair Chillingworth, Theresa Feltwell, refl ects the current lack of consensus as Ahmad Sami Chaouki, Helen K. Zahra Abdellah, Heidi Hauser, to how the major branches of eukaryotes Salz Kay Jagels, Mark Maddison, fi t together. The illustrations from upper Sharon Moule, Mandy Sanders, left to bottom right are as follows: a single Mammalian Small Nucleolar 1984 Sally Whitehead, Michael A. scale from the surface of Umbellosphaera; RNAs Are Mobile Genetic e205 Quail, Gordon Dougan, Julian Amoeba, the large amoeboid organism Elements Parkhill, Michael B. Prentice used as an introduction to protists for Michel J. Weber many school children; Euglena, the iconic Low Levels of Genetic 2052 fl agellate that is often used to challenge Soft Sweeps III: The Signature 1998 Divergence across e215 ideas of plants (Euglena has chloroplasts) of Positive Selection from e186 Geographically and and animals (Euglena moves); Stentor, Recurrent Mutation Linguistically Diverse one of the larger ciliates; Cacatua, the Pleuni S. -

The Closest Unicellular Relatives of Animals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 12, 1773–1778, October 15, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(02)01187-9 The Closest Unicellular Relatives of Animals B.F. Lang,1,2 C. O’Kelly,1,3 T. Nerad,4 M.W. Gray,1,5 Results and Discussion and G. Burger1,2,6 1The Canadian Institute for Advanced Research The evolution of the Metazoa from single-celled protists Program in Evolutionary Biology is an issue that has intrigued biologists for more than a 2 De´ partement de Biochimie century. Early morphological and more recent ultra- Universite´ de Montre´ al structural and molecular studies have converged in sup- Succursale Centre-Ville porting the now widely accepted view that animals are Montre´ al, Que´ bec H3C 3J7 related to Fungi, choanoflagellates, and ichthyosporean Canada protists. However, controversy persists as to the spe- 3 Bigelow Laboratory for Ocean Sciences cific evolutionary relationships among these major P.O. Box 475 groups. This uncertainty is reflected in the plethora of 180 McKown Point Road published molecular phylogenies that propose virtually West Boothbay Harbor, Maine 04575 all of the possible alternative tree topologies involving 4 American Type Culture Collection Choanoflagellata, Fungi, Ichthyosporea, and Metazoa. 10801 University Boulevard For example, a monophyletic MetazoaϩChoanoflagel- Manassas, Virginia 20110 lata group has been suggested on the basis of small 5 Department of Biochemistry subunit (SSU) rDNA sequences [1, 6, 7]. Other studies and Molecular Biology using the same sequences have allied Choanoflagellata Dalhousie University with the Fungi [8], placed Choanoflagellata prior to the Halifax, Nova Scotia B3H 4H7 divergence of animals and Fungi [9], or even placed Canada them prior to the divergence of green algae and land plants [10]. -

Fungal Evolution: Major Ecological Adaptations and Evolutionary Transitions
Biol. Rev. (2019), pp. 000–000. 1 doi: 10.1111/brv.12510 Fungal evolution: major ecological adaptations and evolutionary transitions Miguel A. Naranjo-Ortiz1 and Toni Gabaldon´ 1,2,3∗ 1Department of Genomics and Bioinformatics, Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology, Dr. Aiguader 88, Barcelona 08003, Spain 2 Department of Experimental and Health Sciences, Universitat Pompeu Fabra (UPF), 08003 Barcelona, Spain 3ICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain ABSTRACT Fungi are a highly diverse group of heterotrophic eukaryotes characterized by the absence of phagotrophy and the presence of a chitinous cell wall. While unicellular fungi are far from rare, part of the evolutionary success of the group resides in their ability to grow indefinitely as a cylindrical multinucleated cell (hypha). Armed with these morphological traits and with an extremely high metabolical diversity, fungi have conquered numerous ecological niches and have shaped a whole world of interactions with other living organisms. Herein we survey the main evolutionary and ecological processes that have guided fungal diversity. We will first review the ecology and evolution of the zoosporic lineages and the process of terrestrialization, as one of the major evolutionary transitions in this kingdom. Several plausible scenarios have been proposed for fungal terrestralization and we here propose a new scenario, which considers icy environments as a transitory niche between water and emerged land. We then focus on exploring the main ecological relationships of Fungi with other organisms (other fungi, protozoans, animals and plants), as well as the origin of adaptations to certain specialized ecological niches within the group (lichens, black fungi and yeasts). -

Sex Is a Ubiquitous, Ancient, and Inherent Attribute of Eukaryotic Life
PAPER Sex is a ubiquitous, ancient, and inherent attribute of COLLOQUIUM eukaryotic life Dave Speijera,1, Julius Lukešb,c, and Marek Eliášd,1 aDepartment of Medical Biochemistry, Academic Medical Center, University of Amsterdam, 1105 AZ, Amsterdam, The Netherlands; bInstitute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Sciences, University of South Bohemia, 370 05 Ceské Budejovice, Czech Republic; cCanadian Institute for Advanced Research, Toronto, ON, Canada M5G 1Z8; and dDepartment of Biology and Ecology, University of Ostrava, 710 00 Ostrava, Czech Republic Edited by John C. Avise, University of California, Irvine, CA, and approved April 8, 2015 (received for review February 14, 2015) Sexual reproduction and clonality in eukaryotes are mostly Sex in Eukaryotic Microorganisms: More Voyeurs Needed seen as exclusive, the latter being rather exceptional. This view Whereas absence of sex is considered as something scandalous for might be biased by focusing almost exclusively on metazoans. a zoologist, scientists studying protists, which represent the ma- We analyze and discuss reproduction in the context of extant jority of extant eukaryotic diversity (2), are much more ready to eukaryotic diversity, paying special attention to protists. We accept that a particular eukaryotic group has not shown any evi- present results of phylogenetically extended searches for ho- dence of sexual processes. Although sex is very well documented mologs of two proteins functioning in cell and nuclear fusion, in many protist groups, and members of some taxa, such as ciliates respectively (HAP2 and GEX1), providing indirect evidence for (Alveolata), diatoms (Stramenopiles), or green algae (Chlor- these processes in several eukaryotic lineages where sex has oplastida), even serve as models to study various aspects of sex- – not been observed yet. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

Towards a Management Hierarchy (Classification) for the Catalogue of Life
TOWARDS A MANAGEMENT HIERARCHY (CLASSIFICATION) FOR THE CATALOGUE OF LIFE Draft Discussion Document Rationale The Catalogue of Life partnership, comprising Species 2000 and ITIS (Integrated Taxonomic Information System), has the goal of achieving a comprehensive catalogue of all known species on Earth by the year 2011. The actual number of described species (after correction for synonyms) is not presently known but estimates suggest about 1.8 million species. The collaborative teams behind the Catalogue of Life need an agreed standard classification for these 1.8 million species, i.e. a working hierarchy for management purposes. This discussion document is intended to highlight some of the issues that need clarifying in order to achieve this goal beyond what we presently have. Concerning Classification Life’s diversity is classified into a hierarchy of categories. The best-known of these is the Kingdom. When Carl Linnaeus introduced his new “system of nature” in the 1750s ― Systema Naturae per Regna tria naturae, secundum Classes, Ordines, Genera, Species …) ― he recognised three kingdoms, viz Plantae, Animalia, and a third kingdom for minerals that has long since been abandoned. As is evident from the title of his work, he introduced lower-level taxonomic categories, each successively nested in the other, named Class, Order, Genus, and Species. The most useful and innovative aspect of his system (which gave rise to the scientific discipline of Systematics) was the use of the binominal, comprising genus and species, that uniquely identified each species of organism. Linnaeus’s system has proven to be robust for some 250 years. The starting point for botanical names is his Species Plantarum, published in 1753, and that for zoological names is the tenth edition of the Systema Naturae published in 1758. -
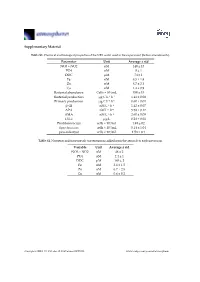
Supplementary Material Parameter Unit Average ± Std NO3 + NO2 Nm
Supplementary Material Table S1. Chemical and biological properties of the NRS water used in the experiment (before amendments). Parameter Unit Average ± std NO3 + NO2 nM 140 ± 13 PO4 nM 8 ± 1 DOC μM 74 ± 1 Fe nM 8.5 ± 1.8 Zn nM 8.7 ± 2.1 Cu nM 1.4 ± 0.9 Bacterial abundance Cells × 104/mL 350 ± 15 Bacterial production μg C L−1 h−1 1.41 ± 0.08 Primary production μg C L−1 h−1 0.60 ± 0.01 β-Gl nM L−1 h−1 1.42 ± 0.07 APA nM L−1 h−1 5.58 ± 0.17 AMA nM L−1·h−1 2.60 ± 0.09 Chl-a μg/L 0.28 ± 0.01 Prochlorococcus cells × 104/mL 1.49 ± 02 Synechococcus cells × 104/mL 5.14 ± 1.04 pico-eukaryot cells × 103/mL 1.58 × 0.1 Table S2. Nutrients and trace metals concentrations added from the aerosols to each mesocosm. Variable Unit Average ± std NO3 + NO2 nM 48 ± 2 PO4 nM 2.4 ± 1 DOC μM 165 ± 2 Fe nM 2.6 ± 1.5 Zn nM 6.7 ± 2.5 Cu nM 0.6 ± 0.2 Atmosphere 2019, 10, 358; doi:10.3390/atmos10070358 www.mdpi.com/journal/atmosphere Atmosphere 2019, 10, 358 2 of 6 Table S3. ANOVA test results between control, ‘UV-treated’ and ‘live-dust’ treatments at 20 h or 44 h, with significantly different values shown in bold. ANOVA df Sum Sq Mean Sq F Value p-value Chl-a 20 H 2, 6 0.03, 0.02 0.02, 0 4.52 0.0634 44 H 2, 6 0.02, 0 0.01, 0 23.13 0.002 Synechococcus Abundance 20 H 2, 7 8.23 × 107, 4.11 × 107 4.11 × 107, 4.51 × 107 0.91 0.4509 44 H 2, 7 5.31 × 108, 6.97 × 107 2.65 × 108, 1.16 × 107 22.84 0.0016 Prochlorococcus Abundance 20 H 2, 8 4.22 × 107, 2.11 × 107 2.11 × 107, 2.71 × 106 7.77 0.0216 44 H 2, 8 9.02 × 107, 1.47 × 107 4.51 × 107, 2.45 × 106 18.38 0.0028 Pico-eukaryote -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -

<I>Mucorales</I>
Persoonia 30, 2013: 57–76 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158513X666259 The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies K. Hoffmann1,2, J. Pawłowska3, G. Walther1,2,4, M. Wrzosek3, G.S. de Hoog4, G.L. Benny5*, P.M. Kirk6*, K. Voigt1,2* Key words Abstract The Mucorales (Mucoromycotina) are one of the most ancient groups of fungi comprising ubiquitous, mostly saprotrophic organisms. The first comprehensive molecular studies 11 yr ago revealed the traditional Mucorales classification scheme, mainly based on morphology, as highly artificial. Since then only single clades have been families investigated in detail but a robust classification of the higher levels based on DNA data has not been published phylogeny yet. Therefore we provide a classification based on a phylogenetic analysis of four molecular markers including the large and the small subunit of the ribosomal DNA, the partial actin gene and the partial gene for the translation elongation factor 1-alpha. The dataset comprises 201 isolates in 103 species and represents about one half of the currently accepted species in this order. Previous family concepts are reviewed and the family structure inferred from the multilocus phylogeny is introduced and discussed. Main differences between the current classification and preceding concepts affects the existing families Lichtheimiaceae and Cunninghamellaceae, as well as the genera Backusella and Lentamyces which recently obtained the status of families along with the Rhizopodaceae comprising Rhizopus, Sporodiniella and Syzygites. Compensatory base change analyses in the Lichtheimiaceae confirmed the lower level classification of Lichtheimia and Rhizomucor while genera such as Circinella or Syncephalastrum completely lacked compensatory base changes. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Protistology Molecular Phylogeny of Aphelidium Arduennense Sp. Nov
Protistology 13 (4), 192–198 (2019) Protistology Molecular phylogeny of Aphelidium arduennense sp. nov. – new representative of Aphelida (Opis- thosporidia) Victoria S. Tcvetkova1, Natalia A. Zorina1, Maria A. Mamkaeva1 and Sergey A. Karpov1,2 1 St. Petersburg State University, St. Petersburg 199034, Russia 2 Zoological Institute, Russian Academy of Sciences, St. Petersburg 199034, Russia | Submitted November 14, 2019 | Accepted December 2, 2019 | Summary Aphelids (Aphelida) are poorly known parasitoids of algae that have raised considerable interest because of their phylogenetic position as phagotrophic protists sister to Fungi. Together with Rozellida and Microsporidia they have been classified in the Opisthosporidia but seem to be more closely related to the Fungi rather than to the Cryptomycota and Microsporidia, the other members of the Opisthosporidia. Molecular environmental studies have revealed high genetic diversity within the aphelids, but only four genera have been described: Aphelidium, Amoeboaphelidium, Paraphelidium and Pseudaphelidium. Here, we describe the life cycle of a new species of Aphelidium, Aph. arduennense. Molecular phylogenetic analysis of its 18S rRNA indicates that Aph. arduennense is sister to Aph. tribonematis, and together with Aph. melosirae they form a monophyletic cluster. Within the aphelids, this cluster is distantly related to Paraphelidium and Amoeboaphelidium. Key words: aphelids, Holomycota, Opisthosporidia, Rozellosporidia, taxonomy Introduction Rozellosporidia (Cryptomycota) formed the super- phylum Opisthosporidia, the deepest branch Aphelids are a divergent group of intracellular of the Holomycota lineage, separated from the parasitoids of green, yellow-green and diatom algae Fungi (Karpov et al., 2014a; Letcher et al., 2015; (Gromov, 2000; Karpov et al., 2014a). The four 2017; Torruella et al., 2015). Several biological known genera have different ecological preferences: peculiarities of the aphelids do not conform the Aphelidium, Amoeboaphelidium and Paraphelidium classical definition of the Fungi. -

Protistology an International Journal Vol
Protistology An International Journal Vol. 10, Number 2, 2016 ___________________________________________________________________________________ CONTENTS INTERNATIONAL SCIENTIFIC FORUM «PROTIST–2016» Yuri Mazei (Vice-Chairman) Welcome Address 2 Organizing Committee 3 Organizers and Sponsors 4 Abstracts 5 Author Index 94 Forum “PROTIST-2016” June 6–10, 2016 Moscow, Russia Website: http://onlinereg.ru/protist-2016 WELCOME ADDRESS Dear colleagues! Republic) entitled “Diplonemids – new kids on the block”. The third lecture will be given by Alexey The Forum “PROTIST–2016” aims at gathering Smirnov (Saint Petersburg State University, Russia): the researchers in all protistological fields, from “Phylogeny, diversity, and evolution of Amoebozoa: molecular biology to ecology, to stimulate cross- new findings and new problems”. Then Sandra disciplinary interactions and establish long-term Baldauf (Uppsala University, Sweden) will make a international scientific cooperation. The conference plenary presentation “The search for the eukaryote will cover a wide range of fundamental and applied root, now you see it now you don’t”, and the fifth topics in Protistology, with the major focus on plenary lecture “Protist-based methods for assessing evolution and phylogeny, taxonomy, systematics and marine water quality” will be made by Alan Warren DNA barcoding, genomics and molecular biology, (Natural History Museum, United Kingdom). cell biology, organismal biology, parasitology, diversity and biogeography, ecology of soil and There will be two symposia sponsored by ISoP: aquatic protists, bioindicators and palaeoecology. “Integrative co-evolution between mitochondria and their hosts” organized by Sergio A. Muñoz- The Forum is organized jointly by the International Gómez, Claudio H. Slamovits, and Andrew J. Society of Protistologists (ISoP), International Roger, and “Protists of Marine Sediments” orga- Society for Evolutionary Protistology (ISEP), nized by Jun Gong and Virginia Edgcomb.