C-Di-AMP: an Essential Molecule in the Signaling Pathways That Regulate the Viability and Virulence of Gram-Positive Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World of Cyclic Dinucleotides in Bacterial Behavior
molecules Review The World of Cyclic Dinucleotides in Bacterial Behavior Aline Dias da Purificação, Nathalia Marins de Azevedo, Gabriel Guarany de Araujo , Robson Francisco de Souza and Cristiane Rodrigues Guzzo * Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, São Paulo 01000-000, Brazil * Correspondence: [email protected] or [email protected]; Tel.: +55-11-3091-7298 Received: 27 December 2019; Accepted: 17 March 2020; Published: 25 May 2020 Abstract: The regulation of multiple bacterial phenotypes was found to depend on different cyclic dinucleotides (CDNs) that constitute intracellular signaling second messenger systems. Most notably, c-di-GMP, along with proteins related to its synthesis, sensing, and degradation, was identified as playing a central role in the switching from biofilm to planktonic modes of growth. Recently, this research topic has been under expansion, with the discoveries of new CDNs, novel classes of CDN receptors, and the numerous functions regulated by these molecules. In this review, we comprehensively describe the three main bacterial enzymes involved in the synthesis of c-di-GMP, c-di-AMP, and cGAMP focusing on description of their three-dimensional structures and their structural similarities with other protein families, as well as the essential residues for catalysis. The diversity of CDN receptors is described in detail along with the residues important for the interaction with the ligand. Interestingly, genomic data strongly suggest that there is a tendency for bacterial cells to use both c-di-AMP and c-di-GMP signaling networks simultaneously, raising the question of whether there is crosstalk between different signaling systems. In summary, the large amount of sequence and structural data available allows a broad view of the complexity and the importance of these CDNs in the regulation of different bacterial behaviors. -

Two-Step Synthesis and Hydrolysis of Cyclic Di-AMP in Mycobacterium Tuberculosis
Two-Step Synthesis and Hydrolysis of Cyclic di-AMP in Mycobacterium tuberculosis Kasi Manikandan1, Varatharajan Sabareesh2¤, Nirpendra Singh3, Kashyap Saigal1, Undine Mechold4, Krishna Murari Sinha1* 1 Institute of Molecular Medicine, New Delhi, India, 2 Council of Scientific and Industrial Research - Institute of Genomics and Integrative Biology, Delhi and IGIB Extension Centre (Naraina), New Delhi, India, 3 Central Instrument Facility, University of Delhi South Campus, New Delhi, India, 4 Institut Pasteur, CNRS UMR 3528, Unite´ de Biochimie des Interactions macromole´culaires, Paris, France Abstract Cyclic di-AMP is a recently discovered signaling molecule which regulates various aspects of bacterial physiology and virulence. Here we report the characterization of c-di-AMP synthesizing and hydrolyzing proteins from Mycobacterium tuberculosis. Recombinant Rv3586 (MtbDisA) can synthesize c-di-AMP from ATP through the diadenylate cyclase activity. Detailed biochemical characterization of the protein revealed that the diadenylate cyclase (DAC) activity is allosterically regulated by ATP. We have identified the intermediates of the DAC reaction and propose a two-step synthesis of c-di-AMP from ATP/ADP. MtbDisA also possesses ATPase activity which is suppressed in the presence of the DAC activity. Investigations by liquid chromatography -electrospray ionization mass spectrometry have detected multimeric forms of c- di-AMP which have implications for the regulation of c-di-AMP cellular concentration and various pathways regulated by the dinucleotide. We have identified Rv2837c (MtbPDE) to have c-di-AMP specific phosphodiesterase activity. It hydrolyzes c-di- AMP to 59-AMP in two steps. First, it linearizes c-di-AMP into pApA which is further hydrolyzed to 59-AMP. -

From Sporulation to Intracellular Offspring Production: Evolution
FROM SPORULATION TO INTRACELLULAR OFFSPRING PRODUCTION: EVOLUTION OF THE DEVELOPMENTAL PROGRAM OF EPULOPISCIUM A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by David Alan Miller January 2012 © 2012 David Alan Miller FROM SPORULATION TO INTRACELLULAR OFFSPRING PRODUCTION: EVOLUTION OF THE DEVELOPMENTAL PROGRAM OF EPULOPISCIUM David Alan Miller, Ph. D. Cornell University 2012 Epulopiscium sp. type B is an unusually large intestinal symbiont of the surgeonfish Naso tonganus. Unlike most other bacteria, Epulopiscium sp. type B has never been observed to undergo binary fission. Instead, to reproduce, it forms multiple intracellular offspring. We believe this process is related to endospore formation, an ancient and complex developmental process performed by certain members of the Firmicutes. Endospore formation has been studied for over 50 years and is best characterized in Bacillus subtilis. To study the evolution of endospore formation in the Firmicutes and the relatedness of this process to intracellular offspring formation in Epulopiscium, we have searched for sporulation genes from the B. subtilis model in all of the completed genomes of members of the Firmicutes, in addition to Epulopiscium sp. type B and its closest relative, the spore-forming Cellulosilyticum lentocellum. By determining the presence or absence of spore genes, we see the evolution of endospore formation in closely related bacteria within the Firmicutes and begin to predict if 19 previously characterized non-spore-formers have the genetic capacity to form a spore. We can also map out sporulation-specific mechanisms likely being used by Epulopiscium for offspring formation. -

Diadenylate Cyclase Evaluation of Ssdaca (SSU98 1483) in Streptococcus Suis Serotype 2
Diadenylate cyclase evaluation of ssDacA (SSU98_1483) in Streptococcus suis serotype 2 B. Du and J.H. Sun Shanghai Key Laboratory of Veterinary Biotechnology, Key Laboratory of Urban Agriculture (South) Ministry of Agriculture, Department of Animal Science, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China Corresponding author: J.H. Sun E-mail: [email protected] Genet. Mol. Res. 14 (2): 6917-6924 (2015) Received December 19, 2014 Accepted February 19, 2015 Published June 18, 2015 DOI http://dx.doi.org/10.4238/2015.June.18.34 ABSTRACT. Cyclic diadenosine monophosphate is a recently identified signaling molecule. It has been shown to play important roles in bacterial pathogenesis. SSU98_1483 (ssDacA), which is an ortholog of Listeria monocytogenes DacA, is a putative diadenylate cyclase in Streptococcus suis serotype 2. In this study, we determined the enzymatic activity of ssDacA in vitro using high-performance liquid chromatography and mass spectrometry. Our results showed that ssDacA was a diadenylate cyclase that converts ATP into cyclic diadenosine monophosphate in vitro. The diadenylate cyclase activity of ssDacA was dependent on divalent metal ions such as Mg2+, Mn2+, or Co2+, and it is more active under basic pH than under acidic pH. The conserved RHR motif in ssDacA was essential for its enzymatic activity, and mutation in this motif abolished the diadenylate cyclase activity of ssDacA. These results indicate that ssDacA is a diadenylate cyclase, which synthesizes cyclic diadenosine monophosphate in Streptococcus suis serotype 2. Key words: Cyclic diadenosine monophosphate; Diadenylate cyclase; DacA; Streptococcus suis serotype 2 Genetics and Molecular Research 14 (2): 6917-6924 (2015) ©FUNPEC-RP www.funpecrp.com.br B. -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -
Supplementary Data TMAO Microbiome R1
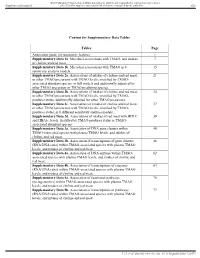
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Deoxyadenosine Triphosphate As a Mediator of Deoxyguanosine Toxicity in Cultured T Lymphoblasts
Deoxyadenosine triphosphate as a mediator of deoxyguanosine toxicity in cultured T lymphoblasts. G J Mann, R M Fox J Clin Invest. 1986;78(5):1261-1269. https://doi.org/10.1172/JCI112710. Research Article The mechanism by which 2'-deoxyguanosine is toxic for lymphoid cells is relevant both to the severe cellular immune defect of inherited purine nucleoside phosphorylase (PNP) deficiency and to attempts to exploit PNP inhibitors therapeutically. We have studied the cell cycle and biochemical effects of 2'-deoxyguanosine in human lymphoblasts using the PNP inhibitor 8-aminoguanosine. We show that cytostatic 2'-deoxyguanosine concentrations cause G1-phase arrest in PNP-inhibited T lymphoblasts, regardless of their hypoxanthine guanine phosphoribosyltransferase status. This effect is identical to that produced by 2'-deoxyadenosine in adenosine deaminase-inhibited T cells. 2'-Deoxyguanosine elevates both the 2'-deoxyguanosine-5'-triphosphate (dGTP) and 2'-deoxyadenosine-5'-triphosphate (dATP) pools; subsequently pyrimidine deoxyribonucleotide pools are depleted. The time course of these biochemical changes indicates that the onset of G1-phase arrest is related to increase of the dATP rather than the dGTP pool. When dGTP elevation is dissociated from dATP elevation by coincubation with 2'-deoxycytidine, dGTP does not by itself interrupt transit from the G1 to the S phase. It is proposed that dATP can mediate both 2'-deoxyguanosine and 2'-deoxyadenosine toxicity in T lymphoblasts. Find the latest version: https://jci.me/112710/pdf Deoxyadenosine Triphosphate as a Mediator of Deoxyguanosine Toxicity in Cultured T Lymphoblasts G. J. Mann and R. M. Fox Ludwig Institute for Cancer Research (Sydney Branch), University ofSydney, Sydney, New South Wales 2006, Australia Abstract urine of PNP-deficient individuals, with elevation of plasma inosine and guanosine and mild hypouricemia (3). -

Gene Conservation Among Endospore-Forming Bacteria Reveals Additional Sporulation Genes in Bacillus Subtilis
Gene Conservation among Endospore-Forming Bacteria Reveals Additional Sporulation Genes in Bacillus subtilis Bjorn A. Traag,a Antonia Pugliese,a Jonathan A. Eisen,b Richard Losicka Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massachusetts, USAa; Department of Evolution and Ecology, University of California Davis Genome Center, Davis, California, USAb The capacity to form endospores is unique to certain members of the low-G؉C group of Gram-positive bacteria (Firmicutes) and requires signature sporulation genes that are highly conserved across members of distantly related genera, such as Clostridium and Bacillus. Using gene conservation among endospore-forming bacteria, we identified eight previously uncharacterized genes that are enriched among endospore-forming species. The expression of five of these genes was dependent on sporulation-specific transcription factors. Mutants of none of the genes exhibited a conspicuous defect in sporulation, but mutants of two, ylxY and ylyA, were outcompeted by a wild-type strain under sporulation-inducing conditions, but not during growth. In contrast, a ylmC mutant displayed a slight competitive advantage over the wild type specific to sporulation-inducing conditions. The phenotype of a ylyA mutant was ascribed to a defect in spore germination efficiency. This work demonstrates the power of combining phy- logenetic profiling with reverse genetics and gene-regulatory studies to identify unrecognized genes that contribute to a con- served developmental process. he formation of endospores is a distinctive developmental the successive actions of four compartment-specific sigma factors Tprocess wherein a dormant cell type (the endospore) is formed (appearing in the order F, E, G, and K), whose activities are inside another cell (the mother cell) and ultimately released into confined to the forespore (F and G) or the mother cell (E and the environment by lysis of the mother cell (1, 2). -

A Metagenomic Window Into the 2-Km-Deep Terrestrial Subsurface Aquifer Revealed Multiple Pathways of Organic Matter Decomposition Vitaly V
FEMS Microbiology Ecology, 94, 2018, fiy152 doi: 10.1093/femsec/fiy152 Advance Access Publication Date: 7 August 2018 Research Article RESEARCH ARTICLE A metagenomic window into the 2-km-deep terrestrial subsurface aquifer revealed multiple pathways of organic matter decomposition Vitaly V. Kadnikov1, Andrey V. Mardanov1, Alexey V. Beletsky1, David Banks3, Nikolay V. Pimenov4, Yulia A. Frank2,OlgaV.Karnachuk2 and Nikolai V. Ravin1,* 1Institute of Bioengineering, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky prosp. 33-2, Moscow, 119071, Russia, 2Laboratory of Biochemistry and Molecular Biology, Tomsk State University, Lenina prosp. 35, Tomsk, 634050, Russia, 3School of Engineering, Systems Power & Energy, Glasgow University, Glasgow G12 8QQ, and Holymoor Consultancy Ltd., 360 Ashgate Road, Chesterfield, Derbyshire S40 4BW, UK and 4Winogradsky Institute of Microbiology, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky prosp 33-2, Moscow, 119071, Russia ∗Corresponding author: Institute of Bioengineering, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky prosp. 33-2, Moscow, 119071, Russia. Tel: +74997833264; Fax: +74991353051; E-mail: [email protected] One sentence summary: metagenome-assembled genomes of microorganisms from the deep subsurface thermal aquifer revealed functional diversity of the community and novel uncultured bacterial lineages Editor: Tillmann Lueders ABSTRACT We have sequenced metagenome of the microbial community of a deep subsurface thermal aquifer in the Tomsk Region of the Western Siberia, Russia. Our goal was the recovery of near-complete genomes of the community members to enable accurate reconstruction of metabolism and ecological roles of the microbial majority, including previously unstudied lineages. The water, obtained via a 2.6 km deep borehole 1-R, was anoxic, with a slightly alkaline pH, and a temperature around 45◦C. -

Mechanistic Studies of the Class I Ribonucleotide Reductase From
Mechanistic Studies of the Class I Ribonucleotide Reductase from Escherichia coli by Erin Jelena Artin Submitted to the Department of Chemistry in partial fulfillment of the requirements for the degree of Doctor of Science in Biochemistry at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2006 c Massachusetts Institute of Technology 2006. All rights reserved. Author.................................................................... Department of Chemistry May 15, 2006 Certified by . Daniel S. Kemp Professor of Chemistry Accepted by............................................................... Robert W. Field Chairman, Department Committee on Graduate Students This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: Professor Daniel S. Kemp . Committee Chair Professor Catherine L. Drennan. Professor Robert G. Griffin . 2 Mechanistic Studies of the Class I Ribonucleotide Reductase from Escherichia coli by Erin Jelena Artin Submitted to the Department of Chemistry on May 15, 2006, in partial fulfillment of the requirements for the degree of Doctor of Science in Biochemistry Abstract Ribonucleotide reductases (RNRs) catalyze the conversion of nucleotides to deoxynucleotides, providing the monomeric precursors required for DNA replication and repair. The class I RNRs are found in many bacteria, DNA viruses, and all eukaryotes including humans, and are composed of two homodimeric subunits: R1 and R2. RNR from Escherichia coli (E. coli) serves as the prototype of this class. R1 has the active site where nucleotide reduc- tion occurs, and R2 contains the diferric-tyrosyl radical (Y · ) cofactor essential for radical initiation on R1. The rate-determining step in E. coli RNR has recently been shown to be a physical step prior to generation of the putative thiyl radical (S · ) on C439. -

First Prebiotic Generation of a Ribonucleotide from Adenine, D– Ribose and Trimetaphosphate
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2011 Supplementary information First Prebiotic Generation of a Ribonucleotide from Adenine, D– Ribose and Trimetaphosphate Graziano Baccolini, a* Carla Boga,a and Gabriele Michelettia a* Department of Organic Chemistry ‘A. Mangini’ ALMA MATER STUDIORUM– Università di Bologna, Viale del Risorgimento 4, 40136 Bologna Italy Fax: (+)39 051 2093654 E-mail: [email protected] Content 1. General Methods 2. Reaction between adenine, D–ribose and trisodium trimetaphosphate 3. Isomerisation of commercial β–adenosine 4. Reaction between guanine, D–ribose and trisodium trimetaphosphate Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2011 1. General Methods Starting reagents (adenine, D-ribose, and trisodium trimetaphosphate) are commercially available, as well as adenosine (9-β-D-Ribofuranosyladenine), adenosine-2’-phosphate hemihydrate (2’- Adenylic acid, 2’-AMP), adenosine-3’-monophosphate (3’-Adenylic acid, 3’-AMP), adenosine-5’- monophosphate (5’-AMP), and adenosine-3’,5’-cyclic monophosphate (3’,5’-AMP), used both as standard reagents for HPLC analyses and as comparison samples to identify the products formed during the reaction. Adenosine 2’,3’-cyclic monophosphate (2’,3’-cAMP) was obtained by acidification of an aqueous solution of its commercial sodium salt. NMR spectra were recorded on Varian Inova 300, Varian Mercury 400 or Varian Inova 600 MHz instruments. Chemical shifts are 1 referenced to external 3-(trimethylsilyl)propionic acid for H and in H2O, and to external standard 31 85% H3PO4 for P NMR. J values are given in Hz. Analytical HPLC analysis was carried out with a Merck-Hitachi Hitachi L-4200 UV-Vis detector on a Supelcosil LC-18-DB 5 μm 250x4.6 mm column at 25 °C. -

Direct Cyclic AMP Enzyme Immunoassay Kit
DetectX® Direct Cyclic AMP Enzyme Immunoassay Kit 1 Plate Kit Catalog Number K019-H1 5 Plate Kit Catalog Number K019-H5 Species Independent Sample Types Validated: Cell Lysates, Saliva, Urine, EDTA and Heparin Plasma, Tissue Culture Media Please read this insert completely prior to using the product. For research use only. Not for use in diagnostic procedures. www.ArborAssays.com K019-H WEB 210301 TABLE OF CONTENTS Background 3 Assay Principle 4 Related Products 4 Supplied Components, Storage Instructions 5 Other Materials Required and Precautions 6 Sample Types 7 Sample Preparation 7-8 Reagent Preparation - General 9 Reagent Preparation - Regular Format 10 Assay Protocol - Regular Format 11 Calc. of Results, Typical & Validation Data - Regular Format 12-13 Acetylated Protocol - Overview 14 Reagent Preparation - Acetylated Format 14-15 Assay Protocol - Acetylated 16 Calc. of Results, Typical & Validation Data - Acetylated Format 17-18 Validation Data - Regular and Acetylated Formats 19-21 Sample Values, Cross Reactivity & Interferents 22 Warranty & Contact Information 23 Plate Layout Sheet 24 ® 2 EXPECT ASSAY ARTISTRY™ K019-H WEB 210301 BACKGROUND Adenosine-3’,5’-cyclic monophosphate, or cyclic AMP (cAMP), C10H12N5O6P, is one of the most important second messengers and a key intracellular regulator. Discovered by Sutherland and Rall in 19571, it functions as a mediator of activity for a number of hormones, including epinephrine, glucagon, and ACTH2-4. Adenylate cyclase is activated by the hormones glucagon and adrenaline and by G protein. Liver adenylate cyclase responds more strongly to glucagon, and muscle adenylate cyclase responds more strongly to adrenaline. cAMP decomposition into AMP is catalyzed by the enzyme phosphodiesterase.