2014 Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amgen and Incyte – the Biotechnology Acquisition
Amgen and Incyte – the biotechnology acquisition Ana Carolina Coelho Student number: 152416013 Dissertation written under the supervision of António Luís Borges de Assunção Dissertation submitted in partial fulfilment of requirements for the MSc in Finance, at the Universidade Católica Portuguesa, May 2018 Abstract The main goal of this dissertation is to study the hypothesis of an acquisition in the biotechnology industry, between Amgen (acquirer) and Incyte (target). Amgen is a U.S.-based biotechnology company currently facing a decrease in sales, mainly due to an increase in the market share of generic drugs and the rise of biosimilar products. To offset the poor performance, it is looking for an acquisition in its industry, for which Incyte could be a suitable target. Incyte belongs to the U.S.-biotechnology industry, and although it has a shy presence in the market with only two released drugs, its revenues are expected to increase significantly in the near future. The combination of these companies would allow knowledge transfer about research and development process of new drugs, a stronger position in Europe, and a higher investment power to apply in R&D. The combined company would be able to decrease the number of employees due to duplication of jobs and decrease the cost of sales, as the power over suppliers increases. The potential synergies are valued at $28,522.8 million, of which $16,739.2 million are more likely to be realized. The combined firm, after the introduction of synergies, is expected to generate the same or even higher returns than the sum of the stand- alone businesses and higher growth rates, fulfilling Amgen’s need of growth. -

XCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) (X) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 ( ) TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 0-19034 REGENERON PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) New York 13-3444607 (State or other jurisdiction of (I.R.S. Employer Identification No) incorporation or organization) 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 (Address of principal executive offices) (Zip code) (914) 347-7000 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None (Title of Class) Securities registered pursuant to Section 12(g) of the Act: Common Stock - par value $.001 per share (Title of Class) Preferred Share Purchase Rights expiring October 18, 2006 (Title of Class) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes /X/ No Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (ss. 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -

Inozyme Pharma Expands Medical Leadership Team
Inozyme Pharma Expands Medical Leadership Team Strengthens Inozyme’s Ability to Advance Lead Candidate, INZ-701, into Clinical Trials Boston, Mass., Nov. 14, 2019 – Inozyme Pharma Inc., a biotechnology company developing novel medicines to treat rare and life-threatening mineralization disorders, today announced the addition of three industry veterans to its leadership team: • Pedro Huertas M.D., Ph.D., as Chief Medical Officer, • Gus Khursigara Ph.D., as Vice President of Medical Affairs and Clinical Operations, and • Catherine Nester, as Vice President of Physician and Patient Strategies. The executives will be instrumental in advancing Inozyme’s lead drug candidate, INZ-701, into clinical trials in 2020 for the treatment of patients with ENPP1 deficiency. INZ-701 is the first therapy that addresses the pathology of ENPP1 deficiency, including diseases such as generalized arterial calcification of infancy (GACI) type 1 and autosomal recessive hypophosphatemic rickets type 2 (ARHR2), both of which are rare and life-threatening manifestations of this enzyme deficiency. Inozyme Pharma received orphan drug designation for INZ-701 in the US and EU in 2018. “We are pleased and excited to welcome Pedro, Gus and Catherine to the Inozyme team,” said Axel Bolte, co-founder and chief executive officer of Inozyme. “Their expertise in orphan drug development – spanning medical affairs, regulatory affairs and clinical development – will strengthen and accelerate our ability to bring potentially life-saving medicines to people who urgently need effective treatments.” Dr. Huertas, a veteran of the pharmaceutical industry, has extensive experience in research and development and medical and regulatory affairs, especially regarding rare disorders and enzyme replacement therapies. -

Virginia: in the Circuit Court of Pittsylvania County
VIRGINIA: IN THE CIRCUIT COURT OF PITTSYLVANIA COUNTY PITTSYLVANIA COUNTY, Plaintiff, v. PURDUE PHARMA, L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; RHODES PHARMACEUTICALS, L.P.; ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC.; MALLINCKRODT PLC; MALLINCKRODT LLC; ENDO HEALTH SOLUTIONS, INC; ENDO PHARMACEUTICALS, INC.; PAR Case No. CL18 - __________ PHARMACEUTICAL COMPANIES, INC.; PAR PHARMACEUTICAL, INC.; TEVA Jury Trial Demanded PHARMACEUTICALS USA, INC.; CEPHALON, INC.; BARR LABORATORIES, INC.; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC.; WATSON LABORATORIES, INC.; ALLERGAN PLC; ACTAVIS PHARMA, INC.; ACTAVIS, LLC; INSYS THERAPEUTICS, INC.; KVK-TECH, INC.; AMNEAL PHARMACEUTICALS LLC; IMPAX LABORATORIES, LLC; AMNEAL PHARMACEUTICALS, INC.; AMNEAL PHARMACEUTICALS OF NEW YORK, LLC; MYLAN PHARMACEUTICALS, INC.; MCKESSON CORPORATION; MCKESSON MEDICAL-SURGICAL INC.; CARDINAL HEALTH, INC.; AMERISOURCEBERGEN DRUG CORPORATION; HENRY SCHEIN, INC.; GENERAL INJECTABLES & VACCINES, INC.; INSOURCE, INC.; CVS HEALTH CORPORATION; CVS PHARMACY, INC.; CVS TN DISTRIBUTION, L.L.C.; WALGREENS BOOTS ALLIANCE, INC.; WALGREEN CO.; EXPRESS SCRIPTS HOLDING COMPANY; EXPRESS SCRIPTS, INC; CAREMARK RX, L.L.C.; CAREMARKPCS HEALTH, L.L.C.; CAREMARK, L.L.C.; UNITEDHEALTH GROUP INCORPORATED; OPTUM, INC.; OPTUMRX, INC.; and DOES 1-100, Defendants. PLAINTIFF’S ORIGINAL COMPLAINT Plaintiff, Pittsylvania County, Virginia, by and through the undersigned attorneys, (hereinafter “Plaintiff,” “Pittsylvania -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -
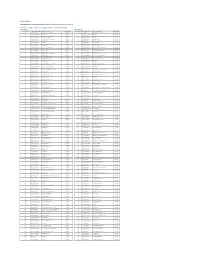
Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN)
Date: 20-Aug-21 Index Weights as of monthly rebalance date 10-Aug-21 Citi Pure Earnings Growth US Long-Short Net TR Index (CIISGRUN) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAP UN Equity Advance Auto Parts Inc 0.24% 1 A UN Equity Agilent Technologies Inc -0.12% 2 ABBV UN Equity AbbVie Inc. 0.59% 2 HWM UN Equity Alcoa Inc -1.02% 3 ABC UN Equity AmerisourceBergen Corp 0.06% 3 AAL UW Equity American Airlines Group Inc -1.09% 4 ADBE UW Equity Adobe Systems Inc 0.01% 4 AAPL UW Equity Apple Inc. -0.46% 5 ADM UN Equity Archer-Daniels-Midland Co 0.26% 5 ABMD UW Equity ABIOMED Inc -0.11% 6 ADSK UW Equity Autodesk Inc 0.26% 6 ABT UN Equity Abbott Laboratories -0.26% 7 AES UN Equity AES Corp 0.37% 7 CB UN Equity ACE Limited -0.07% 8 AIG UN Equity American Intl Group Inc 0.52% 8 ACN UN Equity Accenture plc -0.29% 9 AIZ UN Equity Assurant Inc 0.11% 9 ADI UW Equity Analog Devices Inc -0.13% 10 ALGN UW Equity Align Technology Inc 0.59% 10 ADP UW Equity Automatic Data Processing -0.76% 11 ALL UN Equity Allstate Corp 0.16% 11 AEE UN Equity Ameren Corp -0.24% 12 ALLE UN Equity Allegion PLC 0.34% 12 AEP UW Equity American Electric Power -0.23% 13 AMAT UW Equity Applied Materials Inc 0.59% 13 AFL UN Equity AFLAC Inc -0.29% 14 AMD UW Equity Advanced Micro Devices Inc 1.15% 14 AJG UN Equity ARTHUR J GALLAGHER & CO -0.23% 15 AME UN Equity AMETEK Inc 0.26% 15 AKAM UW Equity Akamai Technologies Inc -0.11% 16 AMT UN Equity American Tower Corp A 0.39% 16 ALB UN -

Complaint, “Chronic Pain” Means Non-Cancer Pain Lasting Three Months Or Longer
TABLE OF CONTENTS Page I. INTRODUCTION ...............................................................................................................1 II. JURISDICTION AND VENUE ..........................................................................................8 III. PARTIES .............................................................................................................................8 A. Plaintiff ....................................................................................................................8 B. Defendants ...............................................................................................................9 IV. FACTUAL ALLEGATIONS ............................................................................................14 A. Defendants Used Multiple Avenues To Disseminate Their False And Deceptive Statements About Opioids. ...................................................................14 1. Defendants spread and continue to spread their false and deceptive statements through direct marketing of their branded opioids. .......................................................................................................15 2. Defendants used a diverse group of seemingly independent third parties to spread false and deceptive statements about the risks and benefits of opioids.................................................................17 a. Key Opinion Leaders (“KOLs”) ....................................................19 (1) Russell Portenoy ................................................................20 -

Amgen 2008 Annual Report and Financial Summary Pioneering Science Delivers Vital Medicines
Amgen 2008 Annual Report and Financial Summary Pioneering science delivers vital medicines About Amgen Products Amgen discovers, develops, manufactures Aranesp® (darbepoetin alfa) and delivers innovative human therapeutics. ® A biotechnology pioneer since 1980, Amgen Enbrel (etanercept) was one of the fi rst companies to realize ® the new science’s promise by bringing EPOGEN (Epoetin alfa) safe and effective medicines from the lab Neulasta® (pegfi lgrastim) to the manufacturing plant to patients. NEUPOGEN® (Filgrastim) Amgen therapeutics have changed the practice of medicine, helping millions of Nplate® (romiplostim) people around the world in the fi ght against ® cancer, kidney disease, rheumatoid Sensipar (cinacalcet) arthritis and other serious illnesses, and so Vectibix® (panitumumab) far, more than 15 million patients worldwide have been treated with Amgen products. With a broad and deep pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people’s lives. 0405 06 07 08 0405 06 07 08 0405 06 07 08 0405 06 07 08 Total revenues “Adjusted” earnings Cash fl ow from “Adjusted” research and ($ in millions) per share (EPS)* operations development (R&D) expenses* (Diluted) ($ in millions) ($ in millions) 2008 $15,003 2008 $4.55 2008 $5,988 2008 $2,910 2007 14,771 2007 4.29 2007 5,401 2007 3,064 2006 14,268 2006 3.90 2006 5,389 2006 3,191 2005 12,430 2005 3.20 2005 4,911 2005 2,302 2004 10,550 2004 2.40 2004 3,697 2004 1,996 * “ Adjusted” EPS and “adjusted” R&D expenses are non-GAAP fi nancial measures. See page 8 for reconciliations of these non-GAAP fi nancial measures to U.S. -

Incyte Corporation 2016 Annual Report
2016 ANNUAL REPORT TABLE OF CONTENTS Letter to Shareholders 2 Key Planned Goals for 2017 6 Innovation 7 Growth 10 Strength 13 Corporate Responsibility 15 Company Information 19 ii Incyte’s Executive Management Team LETTER TO SHAREHOLDERS Standing, left to right: Steven H. Stein, MD; Dear Shareholders, Paula J. Swain; Wenqing Yao, PhD; Jonathan E. Dickinson; At Incyte, we believe that innovation and the discovery of new products David W. Gryska; creates long-term value for patients and society, as well as for our employ- Hervé Hoppenot; Michael Morrissey; ees and our shareholders. It is our commitment to these objectives that has Vijay Iyengar, MD enabled us to make significant progress in the last year. During 2016, we saw continued growth in the number of patients being treated with Jakafi® Seated, left to right: Eric H. Siegel, JD, MBA; (ruxolitinib), our JAK1/JAK2 inhibitor, and we also added Iclusig® (ponati- Reid M. Huber, PhD; nib) to our commercial portfolio as part of our European transaction with Barry P. Flannelly, PharmD, MBA ARIAD Pharmaceuticals, Inc. In February 2017, with Eli Lilly & Company, we announced the European approval of Olumiant® (baricitinib). I believe that we are on Summary of Revenue-Generating Products track to reach our goal of PRODUCT NAME DRUG NAME TARGET APPROVED TERRITORY INDICATION(S) becoming a world-class, Jakafi® ruxolitinib1 JAK1/JAK2 Global MF3; PV4 Jakavi® global biopharmaceutical organization. For the first Iclusig® ponatinib BCR-ABL Europe CML and Ph+ ALL5 time in the history of our company, Incyte’s total Olumiant® baricitinib2 JAK1/JAK2 Europe RA6 yearly revenue surpassed $1 billion in 2016. -

Large Cap Value Fund Q3 Portfolio Holdings
Putnam Equity Income Fund The fund's portfolio 8/31/20 (Unaudited) COMMON STOCKS (95.5%)(a) Shares Value Aerospace and defense (3.0%) Northrop Grumman Corp. 763,179 $261,472,757 Raytheon Technologies Corp. 1,931,815 117,840,715 379,313,472 Airlines (1.0%) Southwest Airlines Co. 3,596,011 135,138,093 135,138,093 Auto components (0.8%) Delphi Automotive PLC 1,165,950 100,411,614 100,411,614 Automobiles (0.7%) General Motors Co. 2,932,830 86,899,753 86,899,753 Banks (9.9%) Bank of America Corp. 13,888,550 357,491,277 Citigroup, Inc. 6,615,251 338,171,631 JPMorgan Chase & Co. 3,413,635 342,012,091 KeyCorp 3,945,705 48,611,086 PNC Financial Services Group, Inc. (The) 1,279,414 142,270,837 1,228,556,922 Beverages (2.5%) Keurig Dr Pepper, Inc.(S) 3,097,349 92,393,921 Molson Coors Beverage Co. Class B(S) 1,663,844 62,627,088 PepsiCo, Inc. 1,123,779 157,396,487 312,417,496 Biotechnology (4.5%) AbbVie, Inc. 2,005,931 192,108,012 Amgen, Inc. 804,202 203,720,451 Regeneron Pharmaceuticals, Inc.(NON) 262,080 162,471,254 558,299,717 Building products (2.3%) Fortune Brands Home & Security, Inc. 1,681,051 141,342,768 Johnson Controls International PLC 3,432,811 139,818,392 281,161,160 Capital markets (3.5%) Apollo Global Management, Inc. 2,289,153 107,292,601 Charles Schwab Corp. (The) 2,516,793 89,421,655 Goldman Sachs Group, Inc. -

Sigma Spectrum Operator's Manual
spectrum Operator’s Manual 35700BAX & 35700ABB Generation 2 Operating System Pump Operating Software v6.05 For Use with MDL Editor v6.2.4 SIGMA, LLC 711 Park Avenue Medina, New York 14103 v 800 356 3454 f 585 798 3909 www.sigmapumps.com Manual 41018- 6.05/6.2.4 Revision D II Pump Operating Software v6.05 For Use With MDL Editor v6.2.4 ©2011 Copyright SIGMA, LLC 711 Park Avenue Medina, New York 14103 v 800 356 3454 f 585 798 3909 www.sigmapumps.com III Manual 41018- 6.05/6.2.4 Revision D IV Pump Operating Software v6.05 For Use With MDL Editor v6.2.4 TABLE OF CONTENTS Introduction and Safety . 1 Intended Device Use . 1 Related Documents . 1 Regulatory Information . 1 Contacting SIGMA Technical Support . 1 Conventions . 2 Summary of Warnings and Cautions . 2 System Components . 11 Spectrum Pump Illustrations . 12 Hardware Labeling . 13 Battery Compatibility . 14 Setting Up the Pump . 15 Unpack the Pump . 15 AC Power Adaptor . 16 Cleaning the Power Adaptor . 16 Connecting the Power Adaptor . 16 Removing the Power Adaptor . 17 Charging the Battery . 17 Configuring User Options . 18 User Options . 18 Operational Overview . 21 Starting a New Infusion Using the Dose Error Reduction System (DERS) . 24 Starting a New Infusion using the BASIC Mode . 25 Secondary Infusions . 26 Preparing the Pump and IV Sets . 27 Loading an IV Set . 28 Unloading an IV Set . 30 Preparing the Pump for a Secondary Infusion . 30 Programming the Pump . 31 Infusion Programming Modes: . 32 Keys Used to Program and Operate the Pump . -

Massmutual Mid Cap Growth Fund T
Fund Holdings As of 06/30/2021 MassMutual Mid Cap Growth Fund T. Rowe Price | Frontier Capital Prior to 5/1/2021, the Fund name was MassMutual Select Mid Cap Growth Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting (%) Amount Value ($) Microchip Technology Inc MCHP 595017104 2.07 1,348,381 201,906,571 Agilent Technologies Inc A 00846U101 1.90 1,252,717 185,164,100 Hologic Inc HOLX 436440101 1.84 2,678,942 178,739,010 Teleflex Inc TFX 879369106 1.82 441,297 177,308,722 Ball Corp BLL 058498106 1.75 2,104,895 170,538,593 Textron Inc TXT 883203101 1.55 2,199,000 151,225,230 Burlington Stores Inc BURL 122017106 1.54 465,929 150,024,479 Catalent Inc CTLT 148806102 1.53 1,376,000 148,773,120 Marvell Technology Inc MRVL 573874104 1.46 2,442,368 142,463,325 Bruker Corp BRKR 116794108 1.39 1,776,000 134,940,480 Ingersoll Rand Inc IR 45687V106 1.29 2,564,000 125,148,840 The Cooper Companies Inc COO 216648402 1.28 314,645 124,684,374 KKR & Co Inc Ordinary Shares KKR 48251W104 1.25 2,050,813 121,490,162 Caesars Entertainment Inc CZR 12769G100 1.13 1,057,315 109,696,431 Reserve Invt Fds 0 76105Y208 1.07 103,877,547 103,877,547 DocuSign Inc DOCU 256163106 1.05 366,000 102,322,620 Chipotle Mexican Grill Inc CMG 169656105 1.04 65,283 101,210,846 Dollar General Corp DG 256677105 1.02 460,009 99,541,348 Veeva Systems Inc Class A VEEV 922475108 1.00 314,189 97,697,070 Avantor Inc AVTR 05352A100 0.98 2,700,000 95,877,000 JB Hunt Transport Services Inc JBHT 445658107 0.98 585,000 95,325,750 KLA Corp KLAC 482480100 0.97 291,710 94,575,299