Comparing the Effect of Gums on the Growth of Lactobacillus Species in Laboratory Medium and Fluid Milk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INULIN and BETA GLUCAN Dr. K. Bhaskarachary
F11FN – Functional Foods and Nutraceuticals F11FN14 - INULIN AND BETA GLUCAN Dr. K. Bhaskarachary Component – I (A) Role Name Affiliation Principal Investigator Dr. Sheela Ramachandran Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore. Co-Principal Investigators Dr. S.Kowsalya Avinashilingam Institute for Home Science Dr.M.Sylvia Subapriya and Higher Education for Women, Dr.G. Bagyalakshmi Coimbatore. Mrs.E.Indira Paper Coordinator Dr. S. Thilakavathy Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore. Content Writer Dr. K. Bhaskarachary Senior Research Officer Dept. Food Chemistry National Institute of Nutrition Jamai Osmania, Hyderabad – 500007. Content Reviewer Dr. K. Bhaskarachary Senior Research Officer Dept. Food Chemistry National Institute of Nutrition Jamai Osmania, Hyderabad – 500007. Language Editor Dr. K. Bhaskarachary Senior Research Officer Dept. Food Chemistry National Institute of Nutrition Jamai Osmania, Hyderabad – 500007. Component-I (B) Description of Module Items Description of Module Subject Name Foods and Nutrition Paper Name Functional Foods and Nutraceuticals Module Name Inulin and Beta Glucan Module ID F11FN14 Pre-requisites physiological benefits of Beta Glucan Objectives • To understand Inulin sources, metabolism and its role in health and food industry • To know the Betaglucan sources, metabolism and its role in various physiological processs and dietary management of non communicable F11FN – Functional Foods and Nutraceuticals F11FN14 - INULIN AND BETA GLUCAN Dr. K. Bhaskarachary diseases and its role in food and health industry Keywords Inulin, non-digestible oligosaccharides, sinistrin, Beta Glucan, prokaryotes and eukaryotes, immunostimulants INTRODUCTION This module explains the structure, metabolism and uses of inulin and beta glucan. The complex carbohydrates of inulin and its dietary fiber properties and their role in physiological process is explained. -

Development of Probiotic Almond Beverage Using Lacticaseibacillus Rhamnosus GR-1 Fortified with Short-Chain and Long-Chain Inulin Fibre
fermentation Article Development of Probiotic Almond Beverage Using Lacticaseibacillus rhamnosus GR-1 Fortified with Short-Chain and Long-Chain Inulin Fibre Lauren Muncey and Sharareh Hekmat * School of Food and Nutritional Sciences, Brescia University College, Western University, London, ON N6G 1H2, Canada; [email protected] * Correspondence: [email protected]; Tel.: +519-432-8353 (ext. 28227) Abstract: Plant-based beverages are growing in popularity due to the rise of vegetarianism and other health trends. A probiotic almond beverage that combines the properties of almonds, inulin, and Lacticaseibacillus rhamnosus GR-1 may meet the demand for a non-dairy health-promoting food. The purpose of this study was to investigate the viability of L. rhamnosus GR-1 and pH in five fermented almond beverage samples, supplemented with either 2% or 5% (w/v) short-chain or long-chain inulin over 9 h of fermentation and 30 days of refrigerated storage. All almond beverage samples achieved a mean viable count of at least 107 CFU/mL during 9h of fermentation and 30 days of refrigerated storage. The probiotic almond beverage supplemented with 2% (w/v) short-chain inulin had a significantly higher mean microbial count (p = 0.048) and lower pH (p < 0.001) throughout fermentation, while the control and the long-chain inulin treatments had the lowest viable counts and acidity, respectively. This study shows that the addition of short-chain and long-chain inulin had Citation: Muncey, L.; Hekmat, S. no adverse effects on the viability of L. rhamnosus GR-1. Therefore, the probiotic almond beverage Development of Probiotic Almond has the potential to be a valid alternative to dairy-based probiotic products. -

The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide As Batch Reaction
Pak. J. Chem. 5(3): 24-30, 2016 Full Paper ISSN (Print): 2220-2625 ISSN (Online): 2222-307X DOI: 10.15228/2016.v06.i01-2.p04 The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide as Batch Reaction *Nahla T. K. Department of Food Sciences, College of Agriculture, University of Baghdad, Iraq E-mail: *[email protected] ABSTRACT Lactulose is a synthetic and non-absorbable disaccharide sugar containing glucose and fructose units. It is used for the treatment of constipation and hepatic encephalopathy. The purpose of this experiment was to synthesized lactulose in the presence of sodium hydroxide as a catalyst. The evaluation of lactulose synthesis was carried out at different concentration of sodium hydroxide (1, 2 and 3M) and the study was also conducted at different temperatures (30, 50, 70, and 90 °C). In addition, the kinetics of lactose isomerization with respect to the time also studied. Finally, it was concluded that the lactulose formation was determined by spectrophotometric method. The lactulose isomerization increased about more than 30.9% at 90 °C in the presence of 1M sodium hydroxide and 20 % lactose concentration. Keywords: Lactose, isomerization, lactulose, sodium hydroxide. 1. INTRODUCTION Lactulose is a synthetic sugar, which is not adsorbed by humans or rodents due to the lack of enzymes which catalyzes the disaccharide sugars. It can be generated by either alkaline isomerization of lactose via the Lobry de Bruyn - Alberda van Ekenstein rearrangement or by enzyme-catalyzed synthesis. Based on the first reaction, different process schemes for the preparation of lactulose have been developed. The enzymatic synthesis of lactulose can be carried out using different pathways with the transgalactosylation reaction being the most promising1. -

Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas1–3
Concentration and distribution of sialic acid in human milk and infant formulas1–3 Bing Wang, Janette Brand-Miller, Patricia McVeagh, and Peter Petocz Downloaded from https://academic.oup.com/ajcn/article/74/4/510/4737459 by guest on 23 September 2021 ABSTRACT 2- to 4-fold higher than those of other mammals, including chim- Background: In animal studies, sialic acid supplementation is panzees (4). Sialic acid is thought to play a role in the structural associated with increases of gangliosides in the brain and and functional establishment of synaptic pathways: >40% of improved learning ability. Only limited data are available on the sialic acid in the brain is found in the synaptosomal fraction and sialic acid content of human milk and infant formulas. contributes to the negative charge of the membrane (3). Because Objective: We compared the concentrations of oligosaccharide- most neurotransmitters are positively charged, sialic acid may bound, protein-bound, and free sialic acid in milk from mothers of assist neurotransmission by facilitating the binding of transmit- full-term and preterm infants and in a range of infant formulas. ter molecules to the synaptic membrane. Design: The milk from 20 and 14 mothers of full-term and Morgan and Winick (2) showed that exogenous sialic acid preterm infants (mean gestational age: 31 ± 3 wk), respectively, administered by intraperitoneal injection increased the produc- was collected at 4 stages of lactation (colostrum, transition, 1 mo, tion of ganglioside sialic acid in the brain and improved learning and 3 mo) and compared with 21 different infant formulas. ability in well-nourished and malnourished rat pups. -

Xorox Univerelty Microfilms
INFORMATION TO USERS This material was producad from a microfilm copy of the original document. While the moit advanced technological meant to photograph and reproduce thii document have been used, the quality it heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help ou understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to ob tain the mining page(s) or section, they are spliced into the film along w ith: adjacent pages, This may have necessitated cutting thru an image and dupli eating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image, fou will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning” the material. It is customary to begin photoi ig at the upper left hand comer of a large dieet and to continue photoi ig from left to right in equal sections with a small overlap. If necessary, sectioning is continued agein — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Lactulose Solution, Usp
LACTULOSE- lactulose solution VistaPharm, Inc ---------- LACTULOSE SOLUTION, USP Rx Only VP2051 04/10 DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g of other sugars). Also contains FD&C Yellow No. 6, purified water, USP and wild cherry flavoring. A minimal quantity of sodium hydroxide, NF is used to adjust pH when necessary. The pH range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation.The chemical name for lactulose is 4-O-β-D- galactopyranosyl-D-fructofuranose. It has the following structural formula: C12H22O11 The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. -
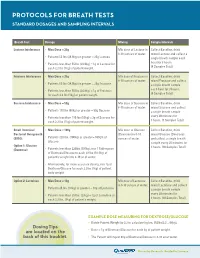
Protocols for Breath Tests Interpretation Help Standard Dosages and Sampling Intervals for Hydrogen/Methane Breath Tests
PROTOCOLS FOR BREATH TESTS INTERPRETATION HELP STANDARD DOSAGES AND SAMPLING INTERVALS FOR HYDROGEN/METHANE BREATH TESTS Breath Test Dosage Mixing Sample Intervals LACTOSE, FRUCTOSE OR SUCROSE INTOLERANCE: Lactose Intolerance • Max Dose = 25g Mix dose of Lactose in Collect Baseline, drink SUSPECTED POSITIVE: 8-10 ounces of water. mixed Lactose and collect a • Hydrogen (H ) Production Only: 20 ppm delta increase in Hydrogen from the lowest preceding value in the test. • Patients 55 lbs (24.9kg) or greater = 25g Lactose single breath sample each 2 hour for 3 hours. • Patients less than 55 lbs (24.9kg) = 1g of Lactose for • Methane (CH4) Production Only: 12 ppm delta increase in Methane from the lowest preceding value in the test. (4 Samples Total) each 2.2 lbs (1kg) of patient weight. • Hydrogen/Methane Both Produced: Add both H2 and CH4 values together in each sample. Review for a 15 ppm delta increase from the lowest preceding value. Fructose Intolerance • Max Dose = 25g Mix dose of Fructose in Collect Baseline, drink 8-10 ounces of water. mixed Fructose and collect • Patients 55 lbs (24.9kg) or greater = 25g Fructose a single breath sample each hour for 3 hours. SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO): • Patients less than 55 lbs (24.9kg) = 1g of Fructose (4 Samples Total) for each 2.2 lbs (1kg) of patient weight. SUSPECTED POSITIVE: Sucrose Intolerance • Max Dose = 50g Mix dose of Sucrose in Collect Baseline, drink OPTION 1: GLUCOSE (DEXTROSE) 8-10 ounces of water. mixed Sucrose and collect • Patients 110 lbs (50kg) or greater = 50g Sucrose • Hydrogen (H ) Production Only: 12 ppm delta increase in a single breath sample 2 every 30 minutes for Hydrogen from the lowest preceding value in the test. -

REVIEW the Role and Potential of Sialic Acid in Human Nutrition
European Journal of Clinical Nutrition (2003) 57, 1351–1369 & 2003 Nature Publishing Group All rights reserved 0954-3007/03 $25.00 www.nature.com/ejcn REVIEW The role and potential of sialic acid in human nutrition B Wang1* and J Brand-Miller1 1Human Nutrition Unit, School of Molecular and Microbial Biosciences, University of Sydney, NSW, Australia Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. In the human body, the highest concentration of sialic acid (as N-acetylneuraminic acid) occurs in the brain where it participates as an integral part of ganglioside structure in synaptogenesis and neural transmission. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides, but its metabolic fate and biological role are currently unknown. An important question is whether the sialic acid in human milk is a conditional nutrient and confers developmental advantages on breast-fed infants compared to those fed infant formula. In this review, we critically discuss the current state of knowledge of the biology and role of sialic acid in human milk and nervous tissue, and the link between sialic acid, breastfeeding and learning behaviour. European Journal of Clinical Nutrition (2003) 57, 1351–1369. doi:10.1038/sj.ejcn.1601704 Keywords: sialic acid; ganglioside; sialyl-oligosaccharides; human milk; infant formula; breastfeeding Introduction promising new candidate is sialic acid (also known as The rapid growth and development of the newborn infant N-acetylneuraminic acid), a nine-carbon sugar that is a puts exceptional demands on the supply of nutrients. -

Impact of Gums on the Growth of Probiotics BERNICE D
Impact of gums on the growth of probiotics BERNICE D. KARLTON-SENAYE*, SALAM. A. IBRAHIM *Corresponding Author North Carolina Agricultural and Technical State University, Bernice D. Food Microbiology and Biotechnology laboratory, Karlton-Senaye Greensboro, NC 27411-1011, USA KEYWORDS: gums; polysaccharides; probiotics; prebiotics ABSTRACT: Gums are polysaccharides used as stabilizers in food that could also enhance growth and viability of probiotics. Thus, the aim of this review was to provide general information about common gums used in different food applications and to introduce a new application of gums as possible functional ingredients to promote the viability of probiotics in food products. INTRODUCTION Molecular structure, chemical composition and functionality of gums Gums are complex polysaccharides extracted from sources Gums are chemically closely related with carbohydrates, such as endosperm of plant seeds, plant exudates, sea but are comprised of cellulose, starches, sugars, oxidation weeds, bacteria, and animal sources (1, 2). Gums are products of these materials, acids, salts of carbon, hydrogen, polymers with hydrophilic ability due to the presence of and oxygen (12). Gums also contain calcium, magnesium, a hydroxyl bond. The composition and structure of gums potassium and sometimes nitrogen (12). Gums can be enable gums to imbibe large amount of water forming a gel, obtained commercially by tapping from certain trees which makes gums useful in the food industry. Gums are used and shrubs, by extracting from marine plants, by milling as stabilizers improving viscosity and texture by preventing or extracting from some seeds, by thermal treatment of Probiotics “wheying off” (3). Gums have also found usefulness in other starches from kernels or root crops, by chemical processing industries, namely pharmaceutical, cosmetic, paint, ink, of cellulose from tree trunks and the cotton plant, as well paper, color, and adhesive industries (4). -

LACTULOSE SOLUTION USP - Lactulose Solution Hi-Tech Pharmacal Co., Inc
LACTULOSE SOLUTION USP - lactulose solution Hi-Tech Pharmacal Co., Inc. DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g or less of other sugars). Lactulose solution contains potassium sorbate as an inactive ingredient. Lactulose is a colonic acidifier which promotes laxation. The chemical name for lactulose is 4-O-β-D-galactopyranosyl-D-fructofuranose. It has the following structural formula: The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours. -

Dietary Fibre Testing
dietary fibres WHAT ARE ANALYSES The Eurofins Carbohydrates Competence Centre routinely performs DIETARY FIBRES? a complete set of tests for total dietary-fibre determination as well as for individual components. If a test is not included in the list below, the Eurofins Carbohydrates Competence Centre also performs specially designed tests for clients. ROUTINE TESTS It is generally recognised that dietary fibre is an essential • Classical total dietary fibre (based on AOAC 985.29) dietary requirement for human beings. In principle, dietary fibre • Classical total, soluble & insoluble dietary fibre (based on is a term that refers to a group of food constituents that pass AOAC 991.43) undigested through the stomach and the small intestine and • Total, high molecular weight (incl. res. starch), low molecular reach the large intestine virtually unchanged. It is made up of weight dietary fibre (based on AOAC 2009.01) indigestible parts of plants and is mainly composed of different • Total, soluble & insoluble (incl. res. starch) high molecular types of non-starch polysaccharides (NSP) and lignin. weight, low molecular weight dietary fibre (based on AOAC 2011.25) It is well documented that dietary fibre is related to health • Total fibre for samples containing supplemented res. benefits such as weight control, satiety, prevention of maltodextrin (based on AOAC 2001.03) constipation, stabilization of blood-glucose levels, reduction • β-glucan (cereals) (based on AOAC 995.16) of cholesterol levels, prevention of certain types of colonic • β -glucan (yeasts & moulds) (only for ingredients or products cancer and prebiotic activity. Nowadays, the demand for with >40% β-glucan content) healthier and less chemically modified food ingredients and • Galactooligosaccharides (based on AOAC 2001.02) foods is becoming more and more important for the consumer. -

Occurrence of Sucrose and Inulin-Hydrolyzing Enzymes In
U. S. DEPARTMENT OF COMMERCE N ATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1526 Part of Journal of Research of the :National Bureau of Standards, Volume 30, March 1943 OCCURRENCE OF SUCROSE AND INULIN.HYDROL YZING ENZYMES IN COMMERCIAL ENZYME PREPARATIONS By William Ward Pigman ABSTRACT The relative enzyme content of enzyme preparations which hydrolyze sucrose and inulin was determined for 14 enzyme preparations representing the principal commercial types available. Those of fungal origin seem invariably to contain invertases in about 0.5 to 1.0 percent of the quantities in commercial purified yeast invertase preparations. Enzyme preparations from plant sources (wheat, almond, and malt), from animal tissues (pancreases), and from Bacillus mesenteri cus had negligible contents of invertase. Enzyme preparations from Aspergillus niger and yeast exhibited considerable ability to hydrolyze inulin, those from Aspergillus oryzae and A. jtavus exhibited some slight activity, and preparations from other sources were essentially inactive. For several representative enzyme preparations, the rate of hydrolysis of inulin and sucrose was studied and found to approximate a first-order reaction. The activity of the inulase in several A. niger enzyme preparations was found to be greatest in the range pH 3 to 4. Invertase preparations from A. niger were most active at pH 3 to 4 and those from A. oryzae at pH 5.0 to 5.5. The results are considered from the viewpoint of the Weidenhagen theory, and it is shown that the original theory is incompatible with the present results in that either the invertases and inulases are different enzymes or they represent a class of enzymes (fructofuranosidases) in which the individual members vary according to the source.