African AIDS Vaccine Programme for a Coordinated and Collaborative Vaccine Development Effort on the Continent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JCRC at 25 Years Magazine
JCRC@25 The birth, growth and evolution of the centre A successful journey that started 25 years ago Testimonies from the beneficiaries What the partners say JCRC @25 Contents eDiToR: Conan Businge Messages (Conan Events Co Ltd) Message from Chairman Board 4 Message from Executive Director 6 DeSign & LAYoUT Diana Kambedha Carol Kabega Features JCRC’s 25-year journey 10 Abou Kisige A story of success and great sacrifice 11 Courtesy Photos cooRDinAToR Fred Byaruhanga Profiles JcRc Board members of the years 8 coMMUnicATionS TeAM 12 Christine Matama Senior management All rights reserved. ©2016 Pictorial Reproduction in whole or in part without written permission is strictly Around JCRC 25 prohibited. JCRC, Plot 101, Lubowa E s t a t e s , O ff E n t e b b e R o a d Partners P.O. Box 10005, Kampala, Uganda UK Medical research Council Tel: 256-414-201147/48 Clinical Trail Unit 31 Fax: 256-414-342632 E-Mail: [email protected] Website: http://www.jcrc.co.ug Interviews With Dr James Makumbi 32 With Dr Justine Jita 33 Research JCRC publications 36 JCRC @25 2 JCRC @25 Contents eDiToR: Conan Businge Messages (Conan Events Co Ltd) Message from Chairman Board 4 Message from Executive Director 6 DeSign & LAYoUT Diana Kambedha Carol Kabega Features JCRC’s 25-year journey 10 Abou Kisige A story of success and great sacrifice 11 Courtesy Photos cooRDinAToR Fred Byaruhanga Profiles JcRc Board members of the years 8 coMMUnicATionS TeAM 12 Christine Matama Senior management All rights reserved. ©2016 Pictorial Reproduction in whole or in part without written permission is strictly Around JCRC 25 prohibited. -

A Media Handbook for HIV Vaccine Trials for Africa Acknowledgements
A Media Handbook for HIV Vaccine Trials for Africa Acknowledgements The Media Handbook for HIV Vaccine Trials for Africa was written by Yinka Adeyemi with guidance and direction from Bunmi Makinwa of the department of Policy, Strategy and Research, Dr Jose Esparza, Dr Saladin Osmanov, Claire Pattou, and Coumba Touré of the World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS), HIV Vaccine Initiative. We would like to acknowledge the following individuals for their valuable comments and contributions to this handbook: Dr Alashle Abimiku, Dr Omu Anzala, Dr Carlos Arnaldo, Dr Courtney Batholomew, Janet Frohlich, Dr D. A. Gangakhedar, Dr Rodney Hoff. Patrick Jabani, Bachi Karkaria, Dr Tom LaSalvia, Dr Chewe Luo, Nebat Mbewe, Dr Rosemary Musonda, Binod Mahanty, Dr Roy Mugerwa, Ronaldo Mussauer de Lima, Omololu Falobi, Otula Owuor, Kirk Pereira, Dr John Rwomushana, Mario Scheffer, Jaya Shreedhar, Judith Soal, Dr Prasert Thongcharoen, Kathy Ann Waterman and Victor Zonana. The section on Communication and vaccine trials in Thailand (Appendix 1) is based on a UNAIDS report by Nusara Thaitawat, while that on Communication issues in vaccine trials in Uganda (Appendix 2) is based on a UNAIDS report by Ann Fieldler. The section on Communication and preparations for HIV vaccine trials in Kenya (Appendix 3) is by Otula Owuor. A number of fictitious people and organizations are used for illustrative purposes within the text. Any reference to actual persons or organizations is purely coincidental. UNAIDS/01.05E (English original, February 2001) ISBN 92-9173-021-1 © Joint United Nations Programme on HIV/AIDS The designations employed and the presentation of the (UNAIDS) 2001. -

Scientific Considerations for the Regulation and Clinical Evaluation of HIV/AIDS Preventive Vaccines
WHO–UNAIDS REPORT Scientific considerations for the regulation and clinical evaluation of HIV/AIDS preventive vaccines Reportà from a WHO-UNAIDS Consultation 13–15 March 2001, Geneva, Switzerland The consultation was jointly organized by the WHO-UNAIDS HIV Vaccine Initiative and the Quality Assurance and Safety of Biologicals Team of the World Health Organization (WHO). Thirty-four experts from 16 developed and developing countries attended the meeting, bringing together expertise from academic institutions, clinical trial centres, national and international regulatory authorities. Representatives of major pharmaceutical companies were also invited. The primary objective of the meeting was to identify gaps that need to be addressed from regulatory perspective to ensure appropriate progress of HIV vaccine develop- ment from basic research to human trials, licensing and future application, with a special focus on needs of developing countries. As a result of discussions, the following priority needs were identified and recommen- dations were made in order to establish an appropriate regulatory framework for the development and evaluation of preventive HIV/AIDS vaccines, which were divided in two main areas: (a) standardization and control of candidate HIV/AIDS vaccines, and (b) approaches to the conduct of clinical trials of candidate HIV/AIDS vaccines.& 2002 Lippincott Williams & Wilkins AIDS 2002, 16:W15–W25 Keywords: HIV/AIDS vaccines, product development, clinical trials, regulatory requirements Introduction the best hope of prevention -

HIV/AIDS and Governance in Uganda and Senegal’ P.I
James Putzel, ‘HIV/AIDS and Governance in Uganda and Senegal’ p.i Institutionalising an Emergency Response: HIV/AIDS and Governance in Uganda and Senegal1 A report submitted to the Department for International Development May 2003 Dr. James Putzel 1 All comments would be welcome by the author: [email protected]. I would like to thank all those in Uganda and Senegal who spared valuable time to meet with me and steer me towards important documentation. I would like to thank all those who provided comments and criticisms on earlier drafts including Tim Allen, Tony Barnett, Charles Becker, Victoria Brittain, Ben Dickson, Garth Glentworth, Julian Lambert, F. Golooba Mutebi, Ken Shadlen, Angela Spilsbury, Sue Unsworth and Alan Whiteside. I am especially grateful to Sarah Hearn for both comments and support throughout this study. Since I have accepted some suggestions and rejected others, I assume full responsibility for what appears in the following pages. James Putzel, ‘HIV/AIDS and Governance in Uganda and Senegal’ p.ii Table of contents Executive Summary................................................................................................. iii Acronyms..................................................................................................................vi 1. Introduction............................................................................................................1 1.1 Why was the international community late?....................................................2 1.2 Why look at governance?.................................................................................3 -

Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Osamah Hamouda, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi
CASCADE Appendix CASCADE Steering Committee : Julia Del Amo (Chair), Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Osamah Hamouda, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi. CASCADE Co-ordinating Centre : Kholoud Porter (Project Leader), Ashley Olson, Kate Coughlin, Sarah Walker, Abdel Babiker. CASCADE Clinical Advisory Board: Heiner C. Bucher, Andrea De Luca, Martin Fisher, Roberto Muga CASCADE Collaborators : Austria: Austrian HIV Cohort Study (Robert Zangerle); Australia PHAEDRA cohort (Tony Kelleher, David Cooper, Pat Grey, Robert Finlayson, Mark Bloch) Sydney AIDS Prospective Study and Sydney Primary HIV Infection cohort (Tony Kelleher, Tim Ramacciotti, Linda Gelgor, David Cooper, Don Smith); Canada South Alberta clinic (John Gill); Estonia Tartu Ülikool (Irja Lutsar); France ANRS CO3 Aquitaine cohort (Geneviève Chêne, Francois Dabis, Rodolphe Thiebaut, Bernard Masquelier), ANRS CO4 French Hospital Database (Dominique Costagliola, Marguerite Guiguet), Lyon Primary Infection cohort (Philippe Vanhems), French ANRS CO6 PRIMO cohort (Marie-Laure Chaix, Jade Ghosn), ANRS CO2 SEROCO cohort (Laurence Meyer, Faroudy Boufassa); Germany German cohort (Osamah Hamouda, Claudia Kücherer, Barbara Bartmeyer); Greece AMACS (Paparizos V, Gargalianos- Kakolyris P, Lazanas M); Greek Haemophilia cohort (Giota Touloumi, Nikos Pantazis, Olga Katsarou); Italy Italian Seroconversion Study (Giovanni Rezza, Maria Dorrucci), ICONA cohort (Antonella d’Arminio Monforte, Andrea De Luca); Netherlands Amsterdam -

Creating National and Regional Frameworks to Support HIV Vaccine Development in Developing Countries
WHO/IVB/05.17 ORIGINAL: ENGLISH Creating national and regional frameworks to support HIV vaccine development in developing countries Report from a WHO-UNAIDS consultation ILausanne, Switzerland, 2–3 September 2004 VImmunization,B Vaccines and Biologicals WHO/IVB/05.17 ORIGINAL: ENGLISH Creating national and regional frameworks to support HIV vaccine development in developing countries Report from a WHO-UNAIDS consultation ILausanne, Switzerland, 2–3 September 2004 VImmunization,B Vaccines and Biologicals The Department of Immunization, Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible. This document was produced by the Initiative for Vaccine Research Team of the Department of Immunization, Vaccines and Biologicals Ordering code: WHO/IVB/ 05.17 Printed: October 2005 This publication is available on the Internet at: www.who.int/vaccines-documents/ Copies may be requested from: World Health Organization Department of Vaccines and Biologicals CH-1211 Geneva 27, Switzerland • Fax: + 41 22 791 4227 • Email: [email protected] • © World Health Organization 2005 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -
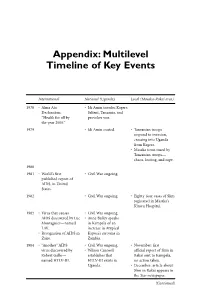
Appendix: Multilevel Timeline of Key Events
Appendix: Multilevel Timeline of Key Events International National (Uganda) Local (Masaka-Rakai area) 1978 • Alma Ata • Idi Amin invades Kagera Declaration: Salient, Tanzania, and “Health for all by provokes war. the year 2000.” 1979 • Idi Amin ousted. • Tanzanian troops respond to invasion, crossing into Uganda from Kagera. • Masaka town razed by Tanzanian troops— chaos, looting, and rape. 1980 1981 • World’s first • Civil War ongoing. published report of AIDS, in United States. 1982 • Civil War ongoing. • Eighty-four cases of Slim registered in Masaka’s Kitovu Hospital. 1983 • Virus that causes • Civil War ongoing. AIDS discovered by Luc • Anne Bailey speaks Montagnier—named in Kampala of an LAV. increase in Atypical • Recognition of AIDS in Kaposi’s sarcoma in Zaire. Zambia. 1984 • “Another” AIDS • Civil War ongoing. • November: first virus discovered by • Wilson Carswell official report of Slim in Robert Gallo— establishes that Rakai sent to Kampala, named HTLV-III. HTLV-III exists in no action taken. Uganda. • December: article about Slim in Rakai appears in the Star newspaper. (Continued) 188 APPENDIX: MULTILEVEL TIMELINE OF KEY EVENTS International National (Uganda) Local (Masaka-Rakai area) 1985 • Civil War ongoing. • February: • Slim the most common investigative team cause of death at from Ministry of Health Mulago national concludes that Slim is referral hospital in typhoid. Kampala. • October: seminal • Ten percent of article published in pregnant women the Lancet detailing Slim attending Mulago in Masaka. Slim and HTLV-III-positive. AIDS said to be different entities. 1986 • Consensus attained • January: Yoweri that LAV and Museveni takes HTLV-III are the power. same virus—renamed • June: blood screening HIV. -

The Experience and Recollections from the Faculties, Schools, Institutes and Centres
8 The Experience and Recollections from the Faculties, Schools, Institutes and Centres Makerere’s Institute of Economics: New Programmes and a Contested Divorce The Harare-based African Capacity Building Foundation (ACBF), had been sponsoring a Masters degree in Economics, which taught African Economics at postgraduate level to assist African governments improve economic policy management for a number of years. McGill University in Montreal, Canada was running the programme for the English speaking African countries on behalf of the ACBF. However, after training a number of African economists at the university for some time, the ACBF was convinced that it made sense to transfer the training to Africa. McGill was not only expensive, it had another disadvantage: students studied in an alien environment, divorced from the realities of African economic problems. This necessitated a search for suitable universities in Anglophone Africa which had the capacity to host the programme. Acting on behalf of the ACBF, McGill University undertook a survey of universities in Anglophone Africa and identified two promising ones which met most of the conditions on ACBF’s checklist for hosting and servicing a regional programme of that kind. Earlier in 1996, Dr Apollinaire Ndorukwigira of the ACBF had visited Makerere to explore the possibility of Makerere participating in the new Economic Policy Management programme. On this particular visit, he said he was not making any commitments because McGill University was yet to undertake a detailed survey of a number of universities in Africa and, based on the findings, McGill University would advise the ACBF on the two most suitable universities which would host the programme. -
Genetic Variability and Consequence of Mycobacterium Tuberculosis Lineage 3 in Kampala-Uganda
RESEARCH ARTICLE Genetic variability and consequence of Mycobacterium tuberculosis lineage 3 in Kampala-Uganda 1,2 1 3 1 Eddie M. WampandeID , Peter Naniima , Ezekiel Mupere , David P. Kateete , LaShaunda L. Malone4,5, Catherine M. Stein5,6, Harriet Mayanja-Kizza4, Sebastien Gagneux7,8, W. Henry Boom5, Moses L. Joloba1* 1 Department of Medical Microbiology, College of Health Sciences, Makerere University, Kampala, Uganda, 2 Department of Veterinary Medicine, Clinical and Comparative medicine, College of Veterinary Medicine, a1111111111 Animal Resources and Bio Security, Makerere University, Kampala, Uganda, 3 Department of Pediatrics and a1111111111 Child Health College of Health Sciences, Makerere University, Kampala, Uganda, 4 Uganda-Case Western a1111111111 Reserve University Research Collaboration, Kampala, Uganda, 5 Tuberculosis Research Unit, School of a1111111111 Medicine, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, OH, Uinted a1111111111 States of America, 6 Department of Population and Quantitative Health Sciences, School of Medicine, Case Western Reserve University, Cleveland, OH, Uinted States of America, 7 Swiss Tropical and Public Health Institute, Basel, Switzerland, 8 University of Basel, Basel, Switzerland * [email protected] OPEN ACCESS Citation: Wampande EM, Naniima P, Mupere E, Abstract Kateete DP, Malone LL, Stein CM, et al. (2019) Genetic variability and consequence of Mycobacterium tuberculosis lineage 3 in Kampala- Uganda. PLoS ONE 14(9): e0221644. https://doi. Background org/10.1371/journal.pone.0221644 Limited data existed exclusively describing Mycobacterium tuberculosis lineage 3 (MTB- Editor: Frederick Quinn, The University of Georgia, L3), sub-lineages, and clinical manifestations in Kampala, Uganda. This study sought to elu- UNITED STATES cidate the circulating MTB-L3 sub-lineages and their corresponding clinical phenotypes. -

Professor Roy D. Mugerwa Remembered by Professor Francis Omaswa with Contributions from Professors Harriet Mayanja and Moses Joloba
Obituary Professor Roy D. Mugerwa Remembered by Professor Francis Omaswa with contributions from Professors Harriet Mayanja and Moses Joloba Professor Roy Deogratius Mugerwa, born 2 Janu- locally at Doctoral, Masters level and others un- ary 1942 and passed on 19 April 2019, is one dertook short courses. Many of the trainees took of the unsung heroes of the health sector and an leadership positions in Makerere and other Uni- academic giant of Makerere University Medical versities, Ministry of Health and other sectors. School. He was one of the patriotic Ugandans They have gone to train others and signifcantly who had opportunities to go and work abroad contribute to the reduction of the epidemic. The during very hard times in Uganda’s history but collaboration has continued to expand beyond decided on several occasions to return to serve HIV/AIDS and had been a model for others. Ugandans after completing higher training Roy Mugerwa was a cardiologist that as- abroad. He contributed signifcantly to the establishment sumed the mantle of leadership when HIV/AIDS emerged of the Uganda Heart Institute and served as Deputy Direc- and led to surges in TB-HIV co-infection. His brilliance, tor to me and later became Director when I moved to the quiet dignity, honesty and elegance were broadly re- Ministry of Health as Director General of Health Services. spected and admired. He advanced the careers of those He initiated echocardiography in Uganda and the frst that succeeded him and hold leadership roles at Maker- specialised hypertension clinic. He founded and was frst ere University, including Harriet-Mayanja-Kizza, Moses Chair of the Uganda Heart Society and I was the Secretary. -
The Politics of Action on Aids: a Case Study of Uganda
public administration and development Public Admin. Dev. 24, 19–30 (2004) Published online in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/pad.306 THE POLITICS OF ACTION ON AIDS: A CASE STUDY OF UGANDA JAMES PUTZEL* London School of Economics and Political Science, London, UK SUMMARY This article examines the political dimensions of Uganda’s progress in bringing a generalised HIV/AIDS epidemic under con- trol. The article documents the history of the political processes involved in Uganda’s battle against HIV/AIDS and analyses the complexities of presidential action and the relation between action at the level of the state and that taken within societal orga- nisations. By the mid-1980s, Uganda was experiencing a full-blown epidemic, the virulence of which was connected with social dislocation and insecurity related to economic crisis and war. Political authorities faced the same challenge as other regimes experiencing the onslaught of AIDS in Africa. The epidemiological characteristics of HIV and AIDS—transmission through heterosexual activities, with a long gestation period, affecting people in the prime of their productive life—meant that action required wide-reaching changes in sexual behaviour, and the educational activities to achieve this, as well as relatively complex systems to monitor the virus and control medical practices (blood supplies, injection practices, mitigating drug delivery). The centralist character of the Museveni regime was crucial not only to mobilising state organisations and foreign aid resources, but also to ensuring significant involvement from non-state associations and religious authorities. The Ugandan experience demon- strates that there is a tension between the requirements for systematic action that a strong public authority can deliver and the need to disseminate information requiring a degree of democratic openness. -

Makerere University's Friends and Development Partners
12 Makerere University’s Friends and Development Partners A Friend in Need If there is such a thing as luck, I have good reasons to believe that good old Makerere had plenty of it. During more than seven decades of its existence as an institution of higher learning, Makerere has been fortunate to attract scores of loyal friends, supporters and well-wishers – individuals and organisations. No doubt, their unwavering support has been its enduring strength. During its darkest chapter in the 1970s and early 1980s, many of its old supporters abandoned it, not so much by choice, but because the prevailing circumstances had made it impossible to continue supporting the institution. As soon as some semblance of peace and political stability returned to the country, one by one, they returned to pick up the pieces. As Professor David Rubadiri (that famous son of Makerere and the former Vice Chancellor of the University of Malawi) once put it, when he visited his old alma mater with his College Principals, Deans, university officials and student leaders in 2001, “During the days of the worst political turmoil Ugandans had been subjected to, the forces of intellectual darkness did everything possible to kill Makerere, but Makerere refused to die.” I believe David Rubadiri summed it up very well. Indeed, Makerere refused to die, because within the dwindling rank and file of its fine academics and seasoned administrators, there were still a few extremely devoted and loyal staff left. They ensured that their beloved institution did not die with Uganda’s turbulent times. It was badly bruised, but refused to go under.