Ntein-Mediated Expression of Cecropin in Escherichia Coli
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vehari, 17/1/1970 Matric 12/07/2014 Younis Ismil Punjab
Renewal List S/NO REN# / NAME FATHER'S NAME PRESENT ADDRESS DATE OF ACADEMIC REN DATE BIRTH QUALIFICATION 1 25303 MUHAMMAD MUHAMMAD CHAK NO. 33/WB, LUDDEN ROAD , VEHARI, 17/1/1970 MATRIC 12/07/2014 YOUNIS ISMIL PUNJAB 2 25586 MUHAMMAD ALAM ABDUL HAMEED CHAK NO. 297 E.B. TEH, BUREWALA DISTT. 14-12- MATRIC 13/07/2014 VEHARI , VEHARI, PUNJAB 1980 3 21990 MUHAMMAD MOHABBAT ALI CHAK NO. 136/EB TEH, BUREWALA DISTT,, 10-10- MATRIC 14/07/2014 SHAFIQ VEHARI, PUNJAB 1980 4 25937 SABA SHAMS QAZI SHAMS UD- VEHARI MAIN ROAD H NO. 231/N MOH, SHARKI 6-7-1983 FA 14/07/2014 DIN COLONY, VEHARI, PUNJAB 5 21418 BASHARAT ALI MUHAMMAD H.NO.27-2 HOUSING SCHEMEBUREWALA, 25/2/1979 MATRIC 14/07/2014 SULEMAN VEHARI, PUNJAB AKHTAR 6 21531 MUHAMMAD RANA USMAN CHAK NO.9/WB, VEHARI, PUNJAB 9/2/1973 MATRIC 14/07/2014 SUBHAN ALI KHAN 7 21508 MUHAMMAD M. AKRAM HASHMI CHOWK NEAR KHANEWAL CHOWK 20/7/1974 MATRIC 14/07/2014 SALEEM 11/W.B, VEHARI, PUNJAB 8 21508 SARDAR SHABIR DILAWAR HOUSE NO. 85/B VEHARI ROAD VEHARI , VEHARI, 2-2-1984 MATRIC 15/07/2014 AKBAR HUSSIAN PUNJAB 9 28179 RABIA GHULAM H/NO. 6679 /-B-1 WARD NO. 2 MOH, RASOOL 15-4-1984 MATRIC 15/07/2014 HUSSAIN PURA MAILSI DISTT, , VEHARI, PUNJAB 10 26928 FAHAD BASHIR ZAHID NAVEED H/NO. 114/CMIAN MARKEET SHARQICOLONY , 12-3-1984 MATRIC 15/07/2014 PASHA VEHARI, PUNJAB 11 30186 MUHAMMAD ALLAH DAWAYA BASTI GHULAM SINDHI P/O FATEH PUR TEH, 15-8-1984 MATRIC 16/07/2014 ZAWAR HUSSAIN MELSI DISTT, VEHARI , VEHARI, PUNJAB 12 33502 MUHAMMAD MUHAMMAD ALI MOH, RIAZ ABAD MULTAN ROAD MELSI DISTT, 3-1-1972 MATRIC 16/7/2014 ASLAM VEHARI , VEHARI, PUNJAB 13 39879 GHULAM ABDUL REHMAN GALI/ MOH, CHAH KUMAR WALA HALEEMKHACHI 1-2-1982 MATRIC 4/8/2014 MURTAZA TEH, MAILSI DISTT,, VEHARI, PUNJAB 14 31433 SABIRA BEGUM MIRZA SHER ALI H NO. -

Institution Wise Pass Percentage SSC 9Th Annual Examination 2017
BOARD OF INTERMEDIATE & SECONDARY EDUCATION, MULTAN A-7 1 INSTITUTION WISE PASS PERCENTAGE AND GRADING 9TH EXAM 2017 Appeared Passed Pass% Appeared Passed Pass% 101001 Govt. Girls English Medium Model 101026 Govt. Girls High School 80/10-R High School, Khanewal Pirowal, Khanewal 787 514 65.31 103 63 61.17 101002 Govt. M.C .E/M Girls High School, 101028 GOVT. GIRLS HIGH SCHOOL BAGAR Khanewal SARGANA, Kabirwala 181 131 72.38 30 27 90.00 101003 Govt. Girls High School, 101029 Govt. Girls High School Nanakpur Kabirwala ,Khanewal 473 207 43.76 44 23 52.27 101004 Govt. Girls Model High School, 101030 Govt. Girls Hassan Model High Mian Channu School, Khanewal. 578 411 71.11 280 179 63.93 101005 Govt. Girls E/M High School, 101031 Govt. Girls High School 92/10.R, Jahanian Khanewal 425 229 53.88 34 25 73.53 101008 Govt. Methodist Girls High School 101032 Govt. Girls High School, 28/10-R 135/16-L, Khanewal ,Khanewal 118 42 35.59 36 33 91.67 101010 Govt. Girls High School 136/10-R, 101033 Govt. Girls High School 23/10-R, Khanewal Khanewal 106 64 60.38 70 40 57.14 101011 Govt. Girls E/M High School, 101034 Govt. Girls High School, 72/10-R 138/10-R, Jahanian ,Khanewal 119 104 87.39 45 13 28.89 101012 Govt. Girls High School, 12/A.H. 101035 Govt. Girls High School, 116/15-L ,Khanewal Mainchannu ,Khanewal 111 77 69.37 33 22 66.67 101013 Govt. Girls High School Sarai 101036 Govt. -

World Bank Document
ENVIRONMENTAL ASSESSMENT (EA) AND THE ENVIRONMENTAL AND SOCIAL MANAGEMENT FRAMEWORK Public Disclosure Authorized PUNJAB EDUCATION SECTOR REFORMS PROGRAM-II (PESRP-II) Public Disclosure Authorized PROGRAM DIRECTOR PUNJAB EDUCATION SECTOR REFORMS PROGRAM (PESRP) SCHOOL EDUCATION DEPARTMENT GOVERNMENT OF THE PUNJAB Tel: +92 42 923 2289~95 Fax: +92 42 923 2290 url: http://pesrp.punjab.gov.pk email: [email protected] Public Disclosure Authorized Revised and Updated for PERSP-II February 2012 Public Disclosure Authorized DISCLAIMER This environmental and social assessment report of the activities of the Punjab Education Sector Reforms Program of the Government of the Punjab, which were considered to impact the environment, has been prepared in compliance to the Environmental laws of Pakistan and in conformity to the Operational Policy Guidelines of the World Bank. The report is Program specific and of limited liability and applicability only to the extent of the physical activities under the PESRP. All rights are reserved with the study proponent (the Program Director, PMIU, PESRP) and the environmental consultant (Environs, Lahore). No part of this report can be reproduced, copied, published, transcribed in any manner, or cited in a context different from the purpose for which it has been prepared, except with prior permission of the Program Director, PESRP. EXECUTIVE SUMMARY This document presents the environmental and social assessment report of the various activities under the Second Punjab Education Sector Reforms Program (PESRP-II) – an initiative of Government of the Punjab for continuing holistic reforms in the education sector aimed at improving the overall condition of education and the sector’s service delivery. -

Tender Notice
NrT A t) 202t I 20 012021 ENLIGHTENINGE f IVES MI]LTAN ELECTRIC POlryER COI,flN Phone fl 061-9330243 oFFrcE oF THE, EXECUTTVE ENGTNEER (CtVtL), CtVtL WORKS DtVtStON MEPCO PBX # 061-9210380-2027 & 2074 NEAR WAPDA HOSPITAL KHANEWAL ROAD MULTAN. TENDER NOTICE. Sr. # Name Work. BOQ. Cost Tender Earnest (Rs) Fee. Monev. (Rs) (Rs) 1 Construction of Office Building (Single Storey) including 86 ,43 ,5251 - 3000i - 2,60,000/- 270 Vehicle Parking shed and compound wall for MEPCO (OP) Sub Division at 1 32 KV Grid Station Kassowal. 2 Construction of 04-No. E-Type (Cat-V) Double Storey Flats 79,21 ,507 t- 3000/- 2,38,000/- 270 at 132 KV Grid Station Sahiwal Old. 3 Construction of Sub Divisional Officer (OP) office building at 70,27,043t- 2500t- 2,11,0001- 210 Jallah Arian (Lodhran). 4 Construction of trench / duct for 11 KV independent feeder in 32 ,21 ,123 t - 2000t- 97,000/- 30 the name of M/S Mehmood Textile Mills Limited lndustrial Estate Multan. Construction / Rehabilitation of Sewerage System to be 21,52,758t- 2000t- 65,000/- bU connected with main sewer line (TMA) of l/EPCO Colony at 132 KV Grid Station Vehari. 6 Construction / Up-raising of transformer way including 21,01,0621- 2000t- 63,500/- 90 culvert, security hut, supply / spreading of water born gravel in switch yard at 132 KV Grid Station Rajan Pur. 7 Re-construction of Trench and Supply / Spreading of Water 18,13,411t- '1500/- 54,500/- bU Born Gravel in switch yard at 132 KV Grid Station Kot Chufta 8 Re-Construction of Switch Yard Fencing (fallen / damaged) 17,46,888/- 1500/- 52,5C0t- 90 and security hut at 132 Grid Station Arifwala. -

Annual Report 2018-19.Pdf
REGIONAL AGRICULTRAL ECONOMIC DEVELOPMENT CENTRE (RAEDC), VEHARI ANNUAL REPORT 2018-19 Dr. GHULAM ABBAS MUSHTAQ ALI FAHAD IHSAN, SAEED AHMAD Seed Farm Road RAEDC Vehari E-mail:[email protected] Web: https://raedc-agripunjab.punjab.gov.pk Ph: 067-3361690, 9201197, Fax: 067-3364428 1 INTRODUCTION Agriculture sector is an integral part of the economy of Pakistan. It is also a strategic priority of other developing countries for balancing export deficits and ensuring food security. To enhance and boost up agricultural production challenges of human resource development and technology transfer play a preeminent role in achievement of objective. Technology transfer mean a „system under which various inter-related components of technology namely “hardware” (materials such as a variety), “software” (technique, know-how, information), “human ware” (human ability), “orgaware” (organizational, management aspects) and the final product (including marketing) are rendered accessible to the end users‟. The pace of technology transfer can only be matched if the farming community is trained/ educated at the same speed. To institutionalize this activity, RAEDC was incepted in 1991. Regional Agricultural Economic Development Centre (RAEDC), Vehari has been organizing courses, which result in capacity building of the officers of the department as well which enhance the capabilities and skills of farmers, unemployed youth, women and labour class particularly related with agriculture. The process involves need assessment of the target populations, demand of the community, unfelt needs of new concepts and technologies. Teaching methods, course contents and training material is very carefully selected with reference to target audience. Teaching methods used in training courses generally involve lecture, field visit, hands-on training, group discussions, brain storming sessions and case studies etc. -

Appendix - II Pakistani Banks and Their Branches (December 31, 2008)
Appendix - II Pakistani Banks and their Branches (December 31, 2008) Allied Bank Ltd. Bhalwal (2) Chishtian (2) -Grain Market -Grain Market (743) -Noor Hayat Colony -Mohar Sharif Road Abbaspur 251 RB Bandla Bheli Bhattar (A.K.) Chitral Chungpur (A.K.) Abbottabad (4) Burewala (2) Dadu -Bara Towers, Jinnahabad -Grain Market -Pineview Road -Housing Scheme Dadyal (A.K) (2) -Supply Bazar -College Road -The Mall Chak Jhumra -Samahni Ratta Cross Chak Naurang Adda Johal Chak No. 111 P Daharki Adda Nandipur Rasoolpur Chak No. 122/JB Nurpur Danna (A.K.) Bhal Chak No. 142/P Bangla Danyor Adda Pansra Manthar Darband Adda Sarai Mochiwal Chak No. 220 RB Dargai Adda Thikriwala Chak No. 272 HR Fortabbas Darhal Gaggan Ahmed Pur East Chak No. 280/JB (Dawakhri) Daroo Jabagai Kombar Akalgarh (A.K) Chak No. 34/TDA Daska Arifwala Chak No. 354 Daurandi (A.K.) Attock (Campbellpur) Chak No. 44/N.B. Deenpur Bagh (A.K) Chak No. 509 GB Deh Uddhi Bahawalnagar Chak No. 76 RB Dinga Chak No. 80 SB Bahawalpur (5) Chak No. 88/10 R Dera Ghazi Khan (2) Chak No. 89/6-R -Com. Area Sattelite Town -Azmat Road -Dubai Chowk -Model Town -Farid Gate Chakwal (2) -Ghalla Mandi -Mohra Chinna Dera Ismail Khan (3) -Settelite Town -Talagang Road -Circular Road -Commissionery Bazar Bakhar Jamali Mori Talu Chaman -Faqirani Gate (Muryali) Balagarhi Chaprar Balakot Charsadda Dhamke (Faisalabad) Baldher Chaskswari (A.K) Dhamke (Sheikhupura) Bucheke Chattar (A.K) Dhangar Bala (A.K) Chhatro (A.K.) Dheed Wal Bannu (2) Dina -Chai Bazar (Ghalla Mandi) Chichawatni (2) Dipalpur -Preedy Gate -College Road Dir Barja Jandala (A.K) -Railway Road Dunyapur Batkhela Ellahabad Behari Agla Mohra (A.K.) Chilas Eminabad More Bewal Bhagowal Faisalabad (20) Bhakkar Chiniot (2) -Akbarabad Bhaleki (Phularwan Chowk) -Muslim Bazar (Main) -Sargodha Road -Chibban Road 415 ABL -Factory Area -Zia Plaza Gt Road Islamabad (23) -Ghulam Muhammad Abad Colony Gujrat (3) -I-9 Industrial Area -Gole Cloth Market -Grand Trunk Road -Aabpara -Gole Kiryana Bazar -Rehman Saheed Road -Blue Area ABL -Gulburg Colony -Shah Daula Road. -

Find Address of Your Nearest Loan Center and Phone Number of Concerned Focal Person
Find address of your nearest loan center and phone number of concerned focal person Loan Center/ S.No. Province District PO Name City / Tehsil Focal Person Contact No. Union Council/ Location Address Branch Name Akhuwat Islamic College Chowk Oppsite Boys College 1 Azad Jammu and Kashmir Bagh Bagh Bagh Nadeem Ahmed 0314-5273451 Microfinance (AIM) Sudan Galli Road Baagh Akhuwat Islamic Muzaffarabad Road Near main bazar 2 Azad Jammu and Kashmir Bagh Dhir Kot Dhir Kot Nadeem Ahmed 0314-5273451 Microfinance (AIM) dhir kot Akhuwat Islamic Mang bajri arja near chambar hotel 3 Azad Jammu and Kashmir Bagh Harighel Harighel Nadeem Ahmed 0314-5273451 Microfinance (AIM) Harighel Akhuwat Islamic 4 Azad Jammu and Kashmir Bhimber Bhimber Bhimber Arshad Mehmood 0346-4663605 Kotli Mor Near Muslim & School Microfinance (AIM) Akhuwat Islamic 5 Azad Jammu and Kashmir Bhimber Barnala Barnala Arshad Mehmood 0346-4663605 Main Road Bimber & Barnala Road Microfinance (AIM) Akhuwat Islamic Main choki Bazar near Sir Syed girls 6 Azad Jammu and Kashmir Bhimber Samahni Samahni Arshad Mehmood 0346-4663605 Microfinance (AIM) College choki Samahni Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 7 Azad Jammu and Kashmir Hattian Hattian UC Hattian Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 8 Azad Jammu and Kashmir Hattian Hattian UC Langla Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Relief and Zahid Hussain HHRD Lamnian office 9 Azad Jammu and Kashmir Hattian Hattian UC Lamnian Zahid Hussain 0345-9071063 Development Main Lamnian Bazar Hattian Bala. -

II Pakistani Schedule Banks Branches As on 31 December 2010
Appendix - II Pakistani Schedule Banks Branches As on 31st December 2010 Allied Bank Ltd. -Noor Hayat Colony -Mohar Sharif Road (806) Bheli Bhattar (A.K.) Chitral Abbaspur 251 RB Bandla Bhiria Chungpur (A.K.) Dadu Abbottabad (4) Burewala (2) -Bara Towers, Jinnahabad -Grain Market Dadyal (A.K) (2) -Pineview Road -Housing Scheme -College Road -Supply Bazar -Samahni Ratta Cross -The Mall Chak Jhumra Chak Naurang Daharki Adda Johal Chak No. 111 P Danna (A.K.) Adda Nandipur Rasoolpur Chak No. 122/JB Nurpur Danyor Bhal Chak No. 142/P Bangla Darband Adda Pansra Manthar Dargai Adda Sarai Mochiwal Chak No. 220 RB Darhal Gaggan Adda Thikriwala Chak No. 272 HR Fortabbas Daroo Jabagai Kombar Alipur Chak No. 280/JB (Dawakhri) Ahmed Pur East Chak No. 34/TDA Daska (2) Akalgarh (A.K) Chak No. 354 -Kutchery road Arifwala Chak No. 44/N.B. -Village budha Attock (Campbellpur) Chak No. 509 GB Bagh (A.K) Chak No. 61 RB Daurandi (A.K.) Bahawalnagar Chak No. 76 RB Deenpur Chak No. 80 SB Deh Uddhi Bahawalpur (5) Chak No. 88/10 R Dinga Chak No. 89/6-R -Com. Area Sattelite Town Chakothi -Dubai Chowk Dera Ghazi Khan (2) -Farid Gate -Azmat Road -Ghalla Mandi Chakwal (3) -Model Town -Settelite Town -Mohra Chinna -Sabzimandi Dera Ismail Khan (3) Bakhar Jamali Mori Talu -Talagang Road -Circular Road Bhawanj -Commissionery Bazar Balagarhi Chaman -Faqirani Gate (Muryali) Balakot Chaprar Baldher Charsadda Dhamke (Faisalabad) Bucheke Chaskswari (A.K) Dhamke (Sheikhupura) Chattar (A.K) Dhangar Bala (A.K) Chhatro (A.K.) Bannu (2) Dheed Wal -Chai Bazar (Ghalla Mandi) Dhudial (Punjab) -Preedy Gate Chichawatni (2) Dina -College Road Dipalpur Barja Jandala (A.K) -Railway Road Dir Batkhela Dunyapur Behari Agla Mohra (A.K.) Chilas Ellahabad Bewal Eminabad More Bhagowal Chiniot (2) Bhakkar -Muslim Bazar (Main) Faisalabad (20) Bhaleki (Phularwan Chowk) -Sargodha Road -Akbarabad -Chibban Road Bhalwal (2) Chishtian (2) -Factory Area -Grain Market -Grain Market 335 -Ghulam Muhammad Abad -Grand Trunk Road -Bara Kahu Colony -Rehman Saheed Road -Blue Area -Gole Cloth Market -Shah Daula Road. -

Population and Household Detail from Block to District Level
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (VEHARI DISTRICT) ADMIN UNIT POPULATION NO OF HH VEHARI DISTRICT 2,897,446 458,068 BUREWALA TEHSIL 1,015,385 160,894 (3-R)BUREWALA QH 126,200 19704 142/E.B. PC 10,562 1555 CHAK NO 138/E.B. 2,181 344 206060401 1,334 214 206060402 847 130 CHAK NO 140/E.B. 2,416 337 206060403 1,716 243 206060404 700 94 CHAK NO 142/E.B. 2,952 446 206060405 1,426 215 206060406 1,526 231 CHAK NO 144/E.B. 3,013 428 206060407 1,213 177 206060408 1,405 194 206060409 395 57 146/E-B PC 15,532 2466 CHAK NO 146/E.B. 6,061 905 206060101 1,567 260 206060102 2,033 247 206060103 1,269 186 206060104 1,192 212 CHAK NO 148/E.B. 3,423 605 206060105 561 90 206060106 1,666 294 206060107 1,196 221 CHAK NO 152/E.B. 3,544 562 206060108 920 163 206060109 996 148 206060110 973 152 206060111 655 99 CHAK NO 170/E.B. 2,504 394 206060112 924 149 206060113 1,080 158 206060114 500 87 150 /E-B PC 4,642 793 CHAK NO 150/E.B. 3,368 590 206060201 1,415 251 206060202 765 139 206060203 1,188 200 CHAK NO 154/E.B. 1,274 203 206060204 1,274 203 156/E-B PC 6,054 971 Page 1 of 79 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (VEHARI DISTRICT) ADMIN UNIT POPULATION NO OF HH CHAK NO 156/E.B. -
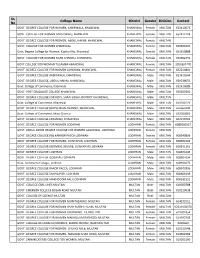
Multan 652410673
Sr. College Name District Gender Division Contact No GOVT. DEGREE COLLEGE FOR WOMEN, KABIRWALA, KHANEWAL KHANEWAL Female MULTAN 652410673 GOVT. COLLEGE FOR WOMEN SARAI SIDHU, KHANEWAL KHANEWAL Female MULTAN 652412228 GOVT. DEGREE COLLEGE FOR WOMEN, ABDUL HAKIM, KHANEWAL KHANEWAL Female MULTAN GOVT. COLLEGE FOR WOMEN KHANEWAL KHANEWAL Female MULTAN 659200107 Govt. Degree College for Women, Katcha Kho, Khanewal KHANEWAL Female MULTAN 652610888 GOVT. COLLEGE FOR WOMEN MIAN CHANNU, KHANEWAL KHANEWAL Female MULTAN 652662293 GOVT.COLLEGE FOR WOMAN TULAMBA KHANEWAL KHANEWAL Female MULTAN 3059367470 GOVT. DEGREE COLLEGE FOR WOMEN JAHANIAN, KHANEWAL KHANEWAL Female MULTAN 652210801 GOVT. DEGREE COLLEGE KABIRWALA, KHANEWAL KHANEWAL Male MULTAN 652410544 GOVT. DEGREE COLLEGE, ABDUL HAKIM, KHANEWAL KHANEWAL Male MULTAN 659239055 Govt. College of Commerce, Kabirwala KHANEWAL Male MULTAN 652410689 GOVT. POST GRADUATE COLLEGE KHANEWAL KHANEWAL Male MULTAN 659200350 GOVT. DEGREE COLLEGE FOR BOYS, SARAI SIDHU, DISTRICT KHANEWAL KHANEWAL Male MULTAN Govt. College of Commerce, Khanewal KHANEWAL Male MULTAN 659200129 GOVT. DEGREE COLLEGE (BOYS) MIAN CHANNU, KHANEWAL KHANEWAL Male MULTAN 652660238 Govt. College of Commerce, Mian Channu KHANEWAL Male MULTAN 652020081 GOVT. DEGREE COLLEGE JAHANIAN, KHANEWAL KHANEWAL Male MULTAN 652210701 GOVT. DEGREE COLLEGE FOR WOMEN LODHRAN LODHRAN Female MULTAN 6089200137 GOVT. ABDUL KARIM DEGREE COLLEGE FOR WOMEN GALAYWAL, LODHRAN LODHRAN Female MULTAN GOVT. DEGREE COLLEGE (W) KAHROR PACCA LODHRAN LODHRAN Female MULTAN 608340836 GOVT. DEGREE COLLEGE FOR WOMEN, DUNYAPUR, LODHRAN LODHRAN Female MULTAN 608304794 GOVT. DEGREE COLLEGE (WOMEN) 365/WB, DUNYAPUR LODHRAN LODHRAN Female MULTAN 608531261 GOVT. DEGREE COLLEGE LODHRAN LODHRAN Male MULTAN 608921046 GOVT. DEGREE COLLEGE GOGRAN, LODHRAN LODHRAN Male MULTAN 608607094 Govt. Commerce College , Lodhran LODHRAN Male MULTAN 608921072 GOVT. -

Police Department District Vehari
ANNUAL POLICING PLAN FOR THE YEAR 2017-18 DISTRICT VEHARI District Police Officer Vehari INTRODUCTION Vehari Police plays an important role in fighting crime protecting people and promoting law and order in District Vehari. The district derives its name from its headquarter town, which was previously Tehsil Headquarter of Multan district. It was created in June, 1976. However, literally Vehari means low lying settlement by a flood water channel. This is also an actual fact as promotion of the district lies along the right bank of the river Sutlij, which forms its southern boundary. Vehari district is situated in the heart of Nili Bar. It is purely the result of construction of Pakpattan Canal from Sulemanki Head Works on the Sutlij and the institution of Nili Bar colony project in 1925, so called because of the bluish tings of the water to the Sutlij. The ancient history of the district is obscure. Populated area in ancient times was restricted to the banks of the river Sutlij where seasonal inundation permitted some cultivation. The rest of the area was a vast sandy scrap- land at beat affording pasture itinerant herdsmen. The river rain tract formed the state of Fatehpur during the time of Akbar the Great. This was ruled by Fateh Khan of Joiya Family who founded and gave his name to the town of Fatehpur. Fatehpur is still in existence about 15 kilometers to the South of Mailsi and is the oldest town of Mailsi sub division. It has some remains of archaeological value. The district lies between 29-36 and 30-22 north latitude and 71-44 and 72-53 longitudes. -

Vehari National Assembly Polling Scheme 2018
ELECTION COMMISSION OF PAKISTAN FORM-28 {See rule 50} LIST OF POLLING STATIONS FOR A CONSTITUENCY Election to the National Assembly Provincial Assembly of the Punjab Sindh Khyber Pakhtunkhwa Balochistan No. and Name of Constituency NA-162 Vehari-I Number of voters assigned to In case of Rural Areas In case of Rural Areas Sr. No. of Voters Number of Polling Booths polling station on the Electoral S. No. Polling Station Name Roll in case electoral Roll is Name of Electoral Census Block Name of Electoral Census Block Male Female Total Male Female Total burificated Area Code Area Code Voters Voters Voters Booths Booths Booths 1 2 3 4 5 6 7 8 9 10 11 12 13 Bhutto Colony (437/EB) Waraich 206100101 412 0 412 Town Govt Commerce College Burewala, 1 Farid Town 206100102 346 0 346 2 0 2 Chungi # 5 Sharif Abad 206100104 117 0 117 Chak505/EB 206100106 101 0 101 TOTAL 976 0 976 Chak 437/EB 206100105 998 0 998 Govt Commerce College Burewala, 2 2 0 2 Chungi # 5 TOTAL 998 0 998 Bhutto Colony (437/EB) Waraich 206100101 0 263 263 Town Govt. Girls Primary. School (Primary Farid Town 206100102 0 289 289 3 0 2 2 Portion) 437/EB (Female)(P) Sharif Abad 206100104 0 85 85 Chak 437/EB 206100105 0 798 798 Chak505/EB 206100106 0 96 96 TOTAL 0 1531 1531 Page 1 of 461 Faiz Abad/Azeem 206120108 375 0 375 Abad Faiz Abad/Azeem 206120110 439 0 439 Abad Vocational Instituite for Women 4 3 0 3 Azeem Abbad(Male)(P) Faiz Abad/Azeem 206120111 398 0 398 Abad Faiz Abad/Azeem 206120112 475 0 475 Abad TOTAL 1687 0 1687 Azeem Abad 206120203 200 0 200 Vocational Instituite for Women