5-HT3A Receptor-Bearing White Matter Interstitial Gabaergic Interneurons Are Functionally Integrated Into Cortical and Subcortical Networks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -
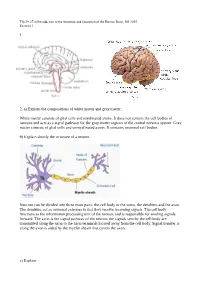
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification
The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit To cite this version: Emmanuel Mandonnet, Silvio Sarubbo, Laurent Petit. The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.94. 10.3389/fnana.2018.00094. hal-01929504 HAL Id: hal-01929504 https://hal.archives-ouvertes.fr/hal-01929504 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 06 November 2018 doi: 10.3389/fnana.2018.00094 The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification Emmanuel Mandonnet 1* †, Silvio Sarubbo 2† and Laurent Petit 3* 1Department of Neurosurgery, Lariboisière Hospital, Paris, France, 2Division of Neurosurgery, Structural and Functional Connectivity Lab, Azienda Provinciale per i Servizi Sanitari (APSS), Trento, Italy, 3Groupe d’Imagerie Neurofonctionnelle, Institut des Maladies Neurodégénératives—UMR 5293, CNRS, CEA University of Bordeaux, Bordeaux, France The heterogeneity and complexity of white matter (WM) pathways of the human brain were discretely described by pioneers such as Willis, Stenon, Malpighi, Vieussens and Vicq d’Azyr up to the beginning of the 19th century. -

Neuroanatomy
Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Neuroanatomy W. Jeffrey Wilson Fall 2012 \Without education we are in a horrible and deadly danger of taking educated people seriously." { Gilbert Keith Chesterton [LATEX in use { a Microsoft- & PowerPoint-free presentation] Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Blood-Brain Barrier Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Peripheral Nervous System • Somatic N.S.: skeletal muscles, skin, joints • Autonomic N.S.: internal organs, glands • Sympathetic N.S.: rapid expenditure of energy • Parasympathetic N.S.: restoration of energy Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Spinal Cord Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain | Ventricles Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Brain Midline Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Hindbrain Myelencephalon & Metencephalon Outline Protection Peripheral Nervous System Overview of Brain Hindbrain Midbrain Forebrain Reticular -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

Neuroimaging of Acute and Chronic Unilateral and Bilateral Thalamic Lesions C
Tuttle et al. Insights into Imaging (2019) 10:24 https://doi.org/10.1186/s13244-019-0700-3 Insights into Imaging PICTORIAL REVIEW Open Access Neuroimaging of acute and chronic unilateral and bilateral thalamic lesions C. Tuttle1†, J. Boto2†, S. Martin1, I. Barnaure2, A. M. Korchi2, M. Scheffler1 and M. I. Vargas2* Abstract The thalami are bilateral ovoid grey matter cerebral structures bordering the third ventricle on both sides, which participate in functions such as relaying of sensory and motor signals, regulation of consciousness, and alertness. Pathologies affecting the thalami can be of neoplastic, infectious, vascular, toxic, metabolic, or congenital origin. The purpose of this review is to provide a comprehensive approach to the thalamus focusing on its anatomy, the main pathologies affecting this structure and their radiological semiology on CT and MRI. We will also illustrate the importance of multimodal MR imaging (morphologic sequences, diffusion-weighted imaging, perfusion, spectroscopy) for the diagnosis and treatment of these conditions. Keywords: Thalami, Magnetic resonance imaging, Computed tomography, Deep brain stimulation, Infection, Neoplasia, Vascular and ischaemic diseases Key points Anatomy and physiology of the thalamus The two thalami are paired ovoid deep brain structures Multimodal MRI plays a crucial role in the detection composed mainly of grey matter, located in the dorsal di- and characterisation of thalamic lesions encephalon together with the epithalami and the metatha- Bilateral thalamic lesions should point to venous lami. In some classifications, the metathalami, also known thrombosis, which is reversible if treated early as the geniculate bodies, are considered part of the thalami. MR imaging is crucial for guiding stereotactic The thalami process and relay information through af- surgical positioning of DBS electrodes ferent and efferent fibers. -

Adult Leukodystrophies: a Step- By-Step Diagnostic Approach
This copy is for personal use only. To order printed copies, contact [email protected] 153 NEUROLOGIC/HEAD A Adult Leukodystrophies: A Step- by-Step Diagnostic Approach Lucas Lopes Resende, MD N Anderson Rodrigues Brandão de Paiva, Leukodystrophies usually affect children, but in the last several D NECK MD decades, many instances of adult leukodystrophies have been re- Fernando Kok, MD, PhD ported in the medical literature. Because the clinical manifestation Claudia da Costa Leite, MD, PhD of these diseases can be nonspecific, MRI can help with establish- Leandro Tavares Lucato, MD, PhD ing a diagnosis. A step-by-step approach to assist in the diagnosis I of adult leukodystrophies is proposed in this article. The first step MAGI Abbreviations: CADASIL = cerebral auto- is to identify symmetric white matter involvement, which is more somal dominant arteriopathy with subcortical commonly observed in these patients. The next step is to fit the infarcts and leukoencephalopathy, CTX = cere- N brotendinous xanthomatosis, FLAIR = fluid-at- symmetric white matter involvement into one of the proposed pat- G tenuated inversion recovery, X-ALD = X-linked terns. However, a patient may present with more than one pattern adrenoleukodystrophy of white matter involvement. Thus, the third step is to evaluate for RadioGraphics 2019; 39:153–168 five distinct characteristics—including enhancement, lesions with https://doi.org/10.1148/rg.2019180081 signal intensity similar to that of cerebrospinal fluid, susceptibility- Content Codes: weighted MRI signal intensity abnormalities, abnormal peaks at MR spectroscopy, and spinal cord involvement—to further narrow From the Neuroradiology Section, Instituto de Radiologia (InRad), Hospital das Clínicas, Facul- the differential diagnosis.