Cochrane Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for NON - CRITICAL CARE Staff Common Vasoactive Drugs These Drugs Are Used to Maintain Cardiovascular Stability
Guidelines for NON - CRITICAL CARE staff Common vasoactive drugs These drugs are used to maintain cardiovascular stability. The full guide to administration can be found on BSUH infonet > intensive care unit > clinical guidelines > inotropes These drugs MUST be given via a central venous catheter. Be given via a ‘dedicated’ line (several vasoactives can run together in one lumen of a CVC) with a 2, 3 or 4 lumen connector. given via an ICU specific syringe driver – these allow you to change the rate without pausing the infusion. All ICU pumps have ICU or HDU spray-painted on them. Be ‘double pumped’ ie: when changing syringe, the old and new infusions run concurrently to prevent loss of infusion during change UNLESS ‘RAPID CHANGE- OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS These drugs MUST NOT be paused, stopped or disconnected suddenly – they have a short half-life and pausing/stopping/disconnection may cause rapid CVS deterioration or arrest UNLESS ‘RAPID CHANGE-OVER’ TECHNIQUE IS USED, DUE TO LACK OF AVAILABLE PUMPS be given as a bolus, or bolused via the pump – this could cause rapid CVS instability run with ANY drug other than a vasoactive drug COMMON VASOACTIVES NORADRENALINE – vasopressor: causes vasoconstriction and used to improve BP USES: sepsis, septic shock, severe hypotension not resolved with fluid ADRENALINE – inotrope: increases contractility, raises BP and HR USES: sepsis, septic shock, severe bradycardia DOBUTAMINE – intrope and vasodilator: increases contractility and reduces cardiac work USES: -

Wednesday, July 10, 2019 4:00Pm
Wednesday, July 10, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – July 10, 2019 DATE: July 3, 2019 NOTE: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the July meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerPsych Program Update – Appendix B Action Item – Vote to Prior Authorize Jornay PM™ [Methylphenidate Extended-Release (ER) Capsule], Evekeo ODT™ [Amphetamine Orally Disintegrating Tablet (ODT)], Adhansia XR™ (Methylphenidate ER Capsule), and Sunosi™ (Solriamfetol Tablet) – Appendix C Action Item – Vote to Prior Authorize Balversa™ (Erdafitinib) – Appendix D Action Item – Vote to Prior Authorize Annovera™ (Segesterone Acetate/Ethinyl Estradiol Vaginal System), Bijuva™ (Estradiol/Progesterone Capsule), Cequa™ (Cyclosporine 0.09% Ophthalmic Solution), Corlanor® (Ivabradine Oral Solution), Crotan™ (Crotamiton 10% Lotion), Gloperba® (Colchicine Oral Solution), Glycate® (Glycopyrrolate Tablet), Khapzory™ (Levoleucovorin Injection), Qmiiz™ ODT [Meloxicam Orally Disintegrating Tablet (ODT)], Seconal Sodium™ (Secobarbital -

FACT SHEET for HEALTHCARE PROVIDERS Coronavirus Emergency Use of the Coviage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19)
FACT SHEET FOR HEALTHCARE PROVIDERS Coronavirus Emergency Use of the COViage System During the COVID-19 Pandemic Disease 2019 September 24, 2020 (COVID-19) This Fact Sheet informs you of the significant known and What do I need to know about COVID-19 treatment? potential risks and benefits of the emergency use of the Current information on COVID-19 infection, including COViage System (or “COViage”) for use by healthcare case definitions and information about clinical signs and providers (HCP) in the hospital setting for adult patients symptoms and/or epidemiological criteria, is available on (18 years of age or older who are admitted to the the CDC website listed at the end of this fact sheet. hospital) with confirmed COVID-19 (based on a positive . PCR test result) for the computation of proprietary patient status indices referred to as Respiratory What is the COViage System? Decompensation Status and Hemodynamic Instability The COViage System is a non-interventional software Status as an adjunct to patient monitoring during the program that uses vital signs and patient demographic COVID-19 outbreak. The COViage indices provide HCP data from EMR systems. It uses models derived from with predictive screening information as a diagnostic aid machine learning on patient data from EMRs to calculate to assist with the early identification of COVID-19 the likelihood of the occurrence of certain clinically patients who are likely to be diagnosed with significant events, specifically, hemodynamic instability hemodynamic instability or respiratory decompensation, or respiratory decompensation. It then notifies HCP of which are common complications associated with patients who, according to the algorithm, are expected to COVID-19. -

The Effects of Dobutamine on Cardiac Sympathetic Activity in Patients with Congestive Heart Failure Abdul Al-Hesayen, MD, Eduardo R
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of the American College of Cardiology Vol. 39, No. 8, 2002 © 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)01783-7 The Effects of Dobutamine on Cardiac Sympathetic Activity in Patients With Congestive Heart Failure Abdul Al-Hesayen, MD, Eduardo R. Azevedo, MD, Gary E. Newton, MD, John D. Parker, MD, FACC Toronto, Canada OBJECTIVES The goal of this work was to study the effects of short-term infusion of dobutamine on efferent cardiac sympathetic activity. BACKGROUND Increased efferent cardiac sympathetic activity is associated with poor outcomes in the setting of congestive heart failure (CHF). Dobutamine is commonly used in the therapy of decompensated CHF. Dobutamine, through its effects on excitatory beta-receptors, may increase cardiac sympathetic activity. METHODS Seven patients with normal left ventricular (LV) function and 13 patients with CHF were studied. A radiotracer technique was used to measure cardiac norepinephrine spillover (CANESP) before and during an intravenous infusion of dobutamine titrated to increase the rate of rise in LV peak positive pressure (ϩdP/dt) by 40%. RESULTS Systemic arterial pulse pressure increased significantly in response to dobutamine in the normal LV function group (74 Ϯ 3mmHgto85Ϯ 3 mm Hg, p ϭ 0.005) but remained unchanged in the CHF group. Dobutamine caused a significant decrease in LV end-diastolic pressure in the CHF group (14 Ϯ 2mmHgto11Ϯ 2 mm Hg, p ϭ 0.02), an effect not observed in the normal LV group. -

Wednesday, June 12, 2019 4:00Pm
Wednesday, June 12, 2019 4:00pm Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Melissa Abbott, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – June 12, 2019 DATE: June 5, 2019 Note: The DUR Board will meet at 4:00pm. The meeting will be held at 4345 N. Lincoln Blvd. Enclosed are the following items related to the June meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Use of Angiotensin Converting Enzyme Inhibitor (ACEI)/ Angiotensin Receptor Blocker (ARB) Therapy in Patients with Diabetes and Hypertension (HTN) Mailing Update – Appendix B Action Item – Vote to Prior Authorize Aldurazyme® (Laronidase) and Naglazyme® (Galsulfase) – Appendix C Action Item – Vote to Prior Authorize Plenvu® [Polyethylene Glycol (PEG)-3350/Sodium Ascorbate/Sodium Sulfate/Ascorbic Acid/Sodium Chloride/Potassium Chloride] – Appendix D Action Item – Vote to Prior Authorize Consensi® (Amlodipine/Celecoxib) and Kapspargo™ Sprinkle [Metoprolol Succinate Extended-Release (ER)] – Appendix E Action Item – Vote to Update the Prior Authorization Criteria For H.P. Acthar® Gel (Repository Corticotropin Injection) – Appendix F Action Item – Vote to Prior Authorize Fulphila® (Pegfilgrastim-jmdb), Nivestym™ (Filgrastim-aafi), -
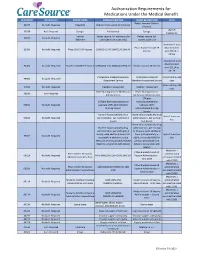
Authorization Requirements for Medications Under
Authorization Requirements for Medications Under the Medical Benefit HCPC/MOD Marketplace BRAND NAME LONG DESCRIPTION SHORT DESCRIPTION Limits Rabies Immune Globulin 90375 No Auth. Required HyperRab Rabies Immune Globulin (Human) (Human) Up to 5 90378 Auth Required Synagis Palivizumab Synagis treatments Imovax Rabies vaccine, for intramuscular Rabies vaccine, for 90675 No Auth. Required Rabavert use (Code price is per 1 mL) intramuscular use Maximum 2 units Pfizer-Biontech Covid-19 Must be billed 91300 No Auth. Required Pfizer COVID-19 Vaccine SARSCOV2 VAC 30MCG/0.3ML IM Vaccine with 0001A or 0002A Maximum 2 units Must be billed 91301 No Auth. Required Moderna COVID-19 Vaccine SARSCOV2 VAC 100MCG/0.5ML IM Moderna Covid-19 Vaccine with 0011A or 0012A Postpartum Maternal Newborn Postpartum Maternal 4 Units Within 180 99501 No Auth. Required Assessment Service Newborn Assessment Service days 4 Units Within 180 99502 No Auth. Required Newborn Assessment Newborn Assessment days Home Nursing Visit for Medication Home Nursing Visit for 99506 Auth Required Administration Medication Administration 17Alpha- 17Alpha-hydroxyprogesterone hydroxyprogesterone 99600 No Auth. Required Caproate (17P) Administration Caproate (17P) Nursing Service Administration Nursing Service Home infusion/specialty drug Home infusion/specialty drug Up to 2 hours per 99601 No Auth. Required administration, per visit (up to 2 administration, per visit (up day hours) to 2 hours) Home infusion/specialty drug Home infusion/specialty drug administration, per visit (up administration, per visit (up to 2 to 2 hours); each additional hours); each additional hour (List hour (List separately in Up to 2 hours per 99602 No Auth. Required separately in addition to code addition to code 99601 for day 99601 for primary procedure) (Use primary procedure) (Use 99602 in conjunction with 99601) 99602 in conjunction with 99601) Maximum 1 Pfizer-Biontech Covid-19 Pfizer COVID-19 Vaccine administration 0001A No Auth. -

Postoperative Vasopressor Usage: a Prospective International Observational Study
SQUEEZE Postoperative vasopressor usage: a prospective international observational study CRF1 PATIENT IDENTIFICATION NUMBER: _____________ PRE-OPERATIVE DATE OF SURGERY: ___________________________ Month and year of birth Sex [m/f] Height [cm] Weight [kg] Clinical Frailty Scale (Rockwood): point 0 to 9. (Will be explained in final CRF) Previous medical history: Coronary Artery Disease: Y/N Cerebrovascular Disease: Y/N Peripheral vascular disease: Y/N Atrial fibrillation: Y/N Heart failure: Y/N Hypertension: Y treated and controlled, Y treated but not controlled, No Diabetes: Takes insulin/managed without insulin/None Chronic liver disease: Y/N Chronic respiratory disease: COPD/other/None Chronic immunosuppression: HIV/other/none Chronic Kidney Disease: No/Yes/Yes and receives renal replacement therapy Long-term steroid use: Y/N Recent/current treatment for cancer (including chemotherapy, radiotherapy, surgery) Regular medications ACE inhibitor: Y and took today/ Y omitted today/N Alpha blocker: Y and took today/ Y omitted today/N Angiotensin Receptor Blocker: Y and took today/ Y omitted today/N Beta blocker: Y and took today/ Y omitted today/N Calcium channel blocker: Y and took today/ Y omitted today Diuretic: Y and took today/ Y omitted today/N Regular NSAIDs: Y/N Regular PPI: Y/N Haemodynamics Measurement in the past 6 months, at least 12h prior to the operating room, at rest: Systolic, Diastolic Heart rate The reading immediately prior to induction of anaesthesia: Systolic, Diastolic Heart rate Laboratory results, most recent (if known -

Vasopressors and Inotropes in Acute Myocardial Infarction Related Cardiogenic Shock: a Systematic Review and Meta-Analysis
Journal of Clinical Medicine Review Vasopressors and Inotropes in Acute Myocardial Infarction Related Cardiogenic Shock: A Systematic Review and Meta-Analysis 1, 2, 1 3 Mina Karami y , Veemal V. Hemradj y, Dagmar M. Ouweneel , Corstiaan A. den Uil , Jacqueline Limpens 4, Luuk C. Otterspoor 5, Alexander P. Vlaar 6 , Wim K. Lagrand 6 and José P. S. Henriques 1,* 1 Heart Center, Department of Interventional Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] (M.K.); [email protected] (D.M.O.) 2 Department of Cardiology, Isala, 8025 AB Zwolle, The Netherlands; [email protected] 3 Departments of Cardiology and Intensive Care Medicine, Erasmus MC, University Medical Center, 3015 GD Rotterdam, The Netherlands; [email protected] 4 Medical Library, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 5 Heart Center Catharina Hospital, 5623 EJ Eindhoven, The Netherlands; [email protected] 6 Department of Intensive Care, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] (A.P.V.); [email protected] (W.K.L.) * Correspondence: [email protected] These two authors contributed equally. y Received: 5 May 2020; Accepted: 25 June 2020; Published: 30 June 2020 Abstract: Vasopressors and inotropes are routinely used in acute myocardial infarction (AMI) related cardiogenic shock (CS) to improve hemodynamics. We aimed to investigate the effect of routinely used vasopressor and inotropes on mortality in AMI related CS. A systematic search of MEDLINE, EMBASE and CENTRAL was performed up to 20 February 2019. -

DOBUTAMINE - Dobutamine Hydrochloride Injection, Solution Physicians Total Care, Inc
DOBUTAMINE - dobutamine hydrochloride injection, solution Physicians Total Care, Inc. ---------- Rx only DESCRIPTION Dobutamine Injection, USP is 1,2-benzenediol, 4-[2-[[3-(4-hydro-xyphenyl)-1- methylpropyl]amino]ethyl]-hydrochloride, (±). It is a synthetic catecholamine. The clinical formulation is supplied in a sterile form for intravenous use only. Each mL contains: Dobutamine hydrochloride, equivalent to 12.5 mg (41.5 µmol) dobutamine; 0.24 mg sodium metabisulfite (added during manufacture), and water for injection. pH adjusted between 2.5 to 5.5 with hydrochloric acid and/or sodium hydroxide. Dobutamine is oxygen sensitive. CLINICAL PHARMACOLOGY Dobutamine is a direct-acting inotropic agent whose primary activity results from stimulation of the β receptors of the heart while producing comparatively mild chronotropic, hypertensive, arrhythmogenic, and vasodilative effects. It does not cause the release of endogenous norepinephrine, as does dopamine. In animal studies, dobutamine produces less increase in heart rate and less decrease in peripheral vascular resistance for a given inotropic effect than does isoproterenol. In patients with depressed cardiac function, both dobutamine and isoproterenol increase the cardiac output to a similar degree. In the case of dobutamine, this increase is usually not accompanied by marked increases in heart rate (although tachycardia is occasionally observed), and the cardiac stroke volume is usually increased. In contrast, isoproterenol increases the cardiac index primarily by increasing the heart rate while stroke volume changes little or declines. Facilitation of atrioventricular conduction has been observed in human electrophysiologic studies and in patients with atrial fibrillation. Systemic vascular resistance is usually decreased with administration of dobutamine. Occasionally, minimum vasoconstriction has been observed. -

PHARMACEUTICAL APPENDIX to the HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev
Harmonized Tariff Schedule of the United States (2008) (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM GADOCOLETICUM 280776-87-6 ABAFUNGIN 129639-79-8 ACIDUM LIDADRONICUM 63132-38-7 ABAMECTIN 65195-55-3 ACIDUM SALCAPROZICUM 183990-46-7 ABANOQUIL 90402-40-7 ACIDUM SALCLOBUZICUM 387825-03-8 ABAPERIDONUM 183849-43-6 ACIFRAN 72420-38-3 ABARELIX 183552-38-7 ACIPIMOX 51037-30-0 ABATACEPTUM 332348-12-6 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABETIMUSUM 167362-48-3 ACIVICIN 42228-92-2 ABIRATERONE 154229-19-3 ACLANTATE 39633-62-0 ABITESARTAN 137882-98-5 ACLARUBICIN 57576-44-0 ABLUKAST 96566-25-5 ACLATONIUM NAPADISILATE 55077-30-0 ABRINEURINUM 178535-93-8 ACODAZOLE 79152-85-5 ABUNIDAZOLE 91017-58-2 ACOLBIFENUM 182167-02-8 ACADESINE 2627-69-2 ACONIAZIDE 13410-86-1 ACAMPROSATE -

PHARMACOLOGY Lecture: Adrenergic Drugs
PHARMACOLOGY Lecture: Adrenergic drugs OBJECTIVES: •Identify the classification of adrenergic agonists •Describe pharmacokinetics and dynamics of adrenergic agonists • Differentiate between the actions of a and b-adrenoceptors agonists • List the clinical uses of adrenergic agonists. • know adverse effects & contraindications of adrenergic agonists • Identify one specific adrenergic agonist for each of the following special uses: Hypotensive states, shock, heart failure, heart decompensation, asthma, premature labour…ect.. Terminology: Chronotropic = increase cardiac output Inotropic = increase cardiac force of contraction Dromotropic = increase conduction velocity Tocolytics (anti-contraction) are medications used to suppress premature labor. Extravasation: leakage of drug from the vessel into tissues surrounding the injection site Adrenaline = epinephrine (E) Noradrenaline (NA) = norepinephrine (NE) . Important. Extra notes. Neurotransmission at adrenergic neurons Adrenergic transmission: Note: Adrenergic neurons 1) Synthesis of norepinephrine release norepinephrine as the 2) Storage of norepinephrine in vesicles primary neurotransmitter. 3) Release of norepinephrine 4) Binding to post synaptic receptors 5) Ending the action of NE by: • Neuronal reuptake into neuron • Monoamine oxidase (MAO) in neuronal mitochondria • Catechol -O-methyl transferase (COMT) in synaptic space Adrenergic receptors Adrenergic receptors Dopaminergic a- b- adrenoceptors adrenoceptors receptors a1 a2 b1 b2 b3 e.g. D1 α1 β2 β1 β3 Post-synaptic excitatory in function (cause inhibitory in function excitatory in In adipose contraction) except in GIT. (cause relaxation) function, present tissue mainly in heart Present mainly in smooth muscles. Contraction of pregnant Relaxation of the uterus (Delay ↑ heart rate: ↑ lipolysis uterus. premature labor) + chronotropic ↑ free effect, Tachycardia fatty acids. Vasoconstriction of skin & Relaxation of skeletal & peripheral blood vessels coronary blood vessels ↑ force of →increased peripheral (vasodilatation) contraction : resistance → hypertension. -

Dobutamine and Isoprenaline After Open Heart Surgery
British Heart Journal, 1976, 38, 622-626. A comparison of cardiovascular effects of dobutamine and isoprenaline after open heart surgery Friedrich Kersting,l Ferenc Follath, Robert Moulds, John Mucklow, Rory McCloy, Joe Sheares, and Colin Dollery From the Departments of Clinical Pharmacology and Surgery, Royal Postgraduate Medical School, Ducane Road, London In a cross-over study, the cardiovascular effects ofdobutamine were assessed in 11 patients who had undergone operation for replacement of the mitral or aortic valve approximately four hours earlier. In 9 of these the effects of isoprenaline were assessedfor comparison. Dose-resporse curves were obtained usingfour dose levels of dobutamine in the range 1-25-10 Vg/kg per min and of isoprenaline in the range of 0 005-0 04 [tg/kg per min. Both drugs produced similar dose-dependent increases in heart rate and cardiac output and a dose- dependent decrease in peripheral resistance. Mean arterial pressure was only slightly increased by either drug. The positive inotropic effect of dobutamine was confirmed, but the chronotropic effect was not significantly different from that of isoprenaline. This contrasts with the findings of previous studies, and possible reasons for this are discussed. One of the most serious sequels of cardiac surgery is naline were compared in 9 patients, the other 2 cardiovascular failure associated with low cardiac receiving dobutamine alone. Only those patients output (Dietzman et al., 1969). Isoprenaline is of in sinus rhythm and free of other postoperative benefit in this situation (Harrison, Kerber, and complications requiring continuous intensive treat- Alderman, 1970) but its use is often limited by the ment were included in the study.