Possible Cytotoxic Activity Analysis of Diethyl Ether Extract of Vaccinium Varingiaefolium (Blume) Miq
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bauhinia Acuminata
299 NON-GENETIC POLYMORPHISM IN BAUHINIA A C U3 'IINA TA L BY K. R. DRONAMRAJU Indian ,5'lalislica[ Institute, 6'alcttlla-.'~5 (Received i0-11-59) INTRODUCTION In the year 1958 Professor J.B.S. I-Ialdane suggested that I should look for heterostylism in Indian plant species where it had not previously been observed. I found a condition resembling it on a bush ofBa:~hinia acuminal,a L. This however differs fi'om the hetero- styIism so far reported in three respects. First, long and short styled flowers are found on the same plant; secondly the lengths of the filaments of the two flower types are not negatively correlated with the style length; and thirdly, most, if not all of the short styled flowers, are female sterile, i began measuring the styles on this bush in tl~e middle of the flowering season, and the results encouraged me to measure them on four other bushes of the same species. A bush can produce up to 50 flowers in a day, so it was possible to compare the results on different bushes, and on the same bush at difl'erent times. ]~ATERIAL The members of the species Bauhblia acumiTmla L. are leguminous plants belonging to the subfamily Caesalpineae. They have woody upright stems growing to a height of 12 feet. The leaves consist of 2 leaEets joined to form a single leaf with two lobes at the apex. The flowers are white and solitary, and very conspicuous, making the bush attractive in a garden. The 5 petals are slightly unequal in size. -
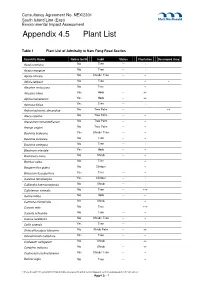
Appendix 4.5 Plant List
Consultancy Agreement No. NEX/2301 South Island Line (East) Environmental Impact Assessment Appendix 4.5 Plant List Table 1 Plant List of Admiralty to Nam Fung Road Section Scientific Name Native to HK Habit Status Plantation Developed Area Acacia confusa No Tree -- + Acacia mangium No Tree -- + Aglaia odorata No Shrub / Tree -- + Albizia lebbeck No Tree -- + + Aleurites moluccana No Tree -- + Alocasia odora Yes Herb -- ++ Alpinia hainanensis Yes Herb -- ++ Aporusa dioica Yes Tree -- + Archontophoenix alexandrae No Tree Palm -- ++ Areca catechu No Tree Palm -- + Arecastrum romanzoffianum No Tree Palm -- + Arenga engleri No Tree Palm -- + Bauhinia blakeana Yes Shrub / Tree -- + Bauhinia purpurea No Tree -- + Bauhinia variegata No Tree -- + Blechnum orientale Yes Herb -- + Boehmeria nivea No Shrub -- + Bombax ceiba No Tree -- + Bougainvillea glabra No Climber -- + Broussonetia papyrifera Yes Tree -- + Calamus tetradactylus Yes Climber -- + Calliandra haematocephala No Shrub -- + Callistemon viminalis No Tree -- +++ Canna indica No Herb -- + Carmona microphylla No Shrub -- + Caryota mitis No Tree -- +++ Caryota ochlandra No Tree -- + Cassia surattensis No Shrub / Tree -- + Celtis sinensis Yes Tree -- + Chrysalidocarpus lutescens No Shrub Palm -- ++ Cinnamomum camphora Yes Tree -- + Codiaeum variegatum No Shrub -- ++ Cordyline fruticosa No Shrub -- ++ Cratoxylum cochinchinense Yes Shrub / Tree -- + Delonix regia No Tree -- + P:\Hong Kong\INF\Projects2\248137 SIL(E) EIA\Deliverables\Final EIA Vol I\3rd\Appendices\4 Ecology\Appendix 4.5 Plant -

Plant Diversity Assessments in Tropical Forest of SE Asia
August 18, 2015, 6th International Barcode of Life Conference Barcodes to Biomes Plant Diversity Assessments in tropical forest of SE Asia Tetsukazu Yahara Center for Asian Conservation Ecology & Institute of Decision Science for a Sustainable Society Kyushu University, Japan Goal: assessing plant species loss under the rapid deforestation in SE Asia Laumonier et al. (2010) Outline • Assessing trends of species richness, PD and community structure in 32 permanent plots of 50m x 50m in Cambodia • Recording status of all the vascular plant species in 100m x 5m plots placed in Vietnam, Cambodia, Thailand, Malaysia and Indonesia • Assessing extinction risks in some representative groups: case studies in Bauhinia and Dalbergia (Fabaceae) Deforestation in Cambodia Sep. 2010 Jan. 2011 Recently, tropical lowland forest of Cambodia is rapidly disappearing; assessments are urgently needed. Locations of plot surveys in Cambodia Unknown taxonomy of plot trees Top et al. (2009); 88 spp (36%) of 243 spp. remain unidentified. Top et al. (2009); many species are mis-identified. Use of DNA barcodes/phylogenetic tree 32 Permanent plots in Kg. Thom 347 species Bayesian method 14 calibration points Estimated common ancestor of Angiosperms 159 Ma 141-199 Ma (Bell et al. 2010) Scientific name: ???? rbcL Local name: Kro Ob Ixonanthes chinensis (544/545) Specimen No.: 2002 Ixonanthes reticulata (556/558) Cyrillopsis paraensis (550/563) Power point slides are prepared for all the plot tree species Scientific name: Ixonanthaceae Ixonanthes reticulata Jack Bokor 240m Local name: Tromoung Sek Phnom matK Ixonanthes chinensis (747/754) Gaps= 0/754 No. 4238 Ixonanthes reticulata (746/754) Gaps= 0/754 # Syn. = Ixonanthes cochinchinensis Pierrei Cyrillopsis paraensis (710/754) Gaps= 0/754“ Ixonanthaceae Ixonanthes reticulata Jack 4238 Specimen image from Kew Herbarium Catalogue http://apps.kew.org/herbcat/gotoHomePage.do Taxonomic papers & Picture Guides Toyama et al. -

World Journal of Pharmaceutical Research Dongray Et Al
World Journal of Pharmaceutical Research Dongray et al. World Journal of Pharmaceutical SJIF ImpactResearch Factor 5.990 Volume 5, Issue 01, 531-546. Review Article ISSN 2277– 7105 PHYTOCHEMICAL AND PHARMACOLOGICAL PROPERTIES OF BAUHINIA ACUMINATA Archana Dongray*, Dr. Raghuveer Irchhaiya, Dilip Chanchal and Saurabh Chaudhary Department of Pharmacognosy, Institute of Pharmacy, Bundelkhand University Jhansi. ABSTRACT Article Received on 09 Nov 2015, Bauhinia species including (Bauhinia acuminata, Bauhinia Revised on 30 Nov 2015, varigata, Bauhinia purpurea, Bauhinia monandra, Bauhinia galpini) Accepted on 23 Dec 2015 are popular ornamental plants usually woody ornamentals or herbaceous linas with attractive flowers typical of the *Correspondence for leguminosae of arid temp. sub – tropical and tropical zones . Author Bauhinia species are also have many multiple medicinal and Archana Dongray biological properties . Phytochemical screening of two species viz . Department of Pharmacognosy, Institute Bauhinia acuminata and cassia occidentailis belonging to family of Pharmacy, Bundelkhand caesalpiniaceae was performed using genrally accepted laboratory University Jhansi. technique . Three solvent viz. chloroform, Benzen, and petrolium ether were used for extraction. The constituents were alkaloids, flavonoids, glycosides, saponin, steroids, and tannin. The distribution of these constituents in the leaves of selected species were assessed and compared . Preliminary phytochemical screening of Bauhinia acuminata did not reveal alkaloids. Glycoside, steroid, and flavonoids were present in both of species . Tannin was present in Cassia occidentalis while absent in Bauhinia acuminata . Saponin was absent in Cassia occidentailis while persent in Bauhinia acuminata . The extraction of leave of Bauhinia acuminata and its kupchan fraction were screend for antioxidant , cytotoxic, membrane stabilizing, hemolytic and antimicrobial activity . KEYWORDS: Bauhinia acuminata, Pharmacognosy, Phytochemicals, and Pharmacology. -

Assessment of Antioxidant and Anti-Inflammatory Activities of Stem Bark of Bauhinia Acuminata L
Research Article ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2020.24.004101 Assessment of Antioxidant and Anti-inflammatory Activities of Stem Bark of Bauhinia acuminata L. Sanjay Dutta2, Sanowar Hossain3, Ekramul Islam1, Uzzal Haque1 and Shahnaj Parvin*1 1Deptment of Pharmacy, University of Rajshahi, Rajshahi-6205, Bangladesh 2Department of Pharmacy, Khwaja Yunus Ali University, Sirajganj, Bangladesh 3Department of Pharmacy, Pabna University of Science and Technology, Pabna, Bangladesh *Corresponding author: Shahnaj Parvin, Department of Pharmacy, University of Rajshahi, Rajshahi-6205, Bangladesh ARTICLE INFO Abstract Received: January 10, 2020 Published: January 21, 2020 Medicinal plants have been used from prehistoric times as first source of health Bauhiniacare to fight acuminata infectious is employed and non ininfectious the treatment diseases. of glandularMost of theseswelling, medicinal skin diseases plants Citation: Sanjay Dutta, Sanowar Hossain, andmay ulcerhave etc.scientific The aim evidence of this towork be wasconsidered to investigate in general the antioxidantpractice. Various capacities parts and of Ekramul Islam, Uzzal Haque, Shahnaj B. acuminata and its different fractions. Crude Methanol Extract (CME) of stems and its various fractions Parvin. Assessment of Antioxidant and suchanti-inflammatory as Chloroform activities (CHF), Ethyl of the Acetate methanol (EF), extract and Aqueous of stem bark(AQF) of were subjected to assay for antioxidant activity using various methods of assay like 2, 2-Di Phenyl Picryl of Bauhinia acuminata L. Biomed J Sci & Hydrazyl (DPPH), hydrogen peroxide and hydroxyl radical-scavenging activity. Total Anti-inflammatory Activities of Stem Bark Tech Res 24(5)-2020. BJSTR. MS.ID.004101. Keywords: Anti-oxidant; Stem Bark; Free pawphenolics edema and model. flavonoids were determined by Folin-Ciocalteu and colorimetric methods. -

Check List Lists of Species Check List 11(4): 1718, 22 August 2015 Doi: ISSN 1809-127X © 2015 Check List and Authors
11 4 1718 the journal of biodiversity data 22 August 2015 Check List LISTS OF SPECIES Check List 11(4): 1718, 22 August 2015 doi: http://dx.doi.org/10.15560/11.4.1718 ISSN 1809-127X © 2015 Check List and Authors Tree species of the Himalayan Terai region of Uttar Pradesh, India: a checklist Omesh Bajpai1, 2, Anoop Kumar1, Awadhesh Kumar Srivastava1, Arun Kumar Kushwaha1, Jitendra Pandey2 and Lal Babu Chaudhary1* 1 Plant Diversity, Systematics and Herbarium Division, CSIR-National Botanical Research Institute, 226 001, Lucknow, India 2 Centre of Advanced Study in Botany, Banaras Hindu University, 221 005, Varanasi, India * Corresponding author. E-mail: [email protected] Abstract: The study catalogues a sum of 278 tree species and management, the proper assessment of the diversity belonging to 185 genera and 57 families from the Terai of tree species are highly needed (Chaudhary et al. 2014). region of Uttar Pradesh. The family Fabaceae has been The information on phenology, uses, native origin, and found to exhibit the highest generic and species diversity vegetation type of the tree species provide more scope of with 23 genera and 44 species. The genus Ficus of Mora- such type of assessment study in the field of sustainable ceae has been observed the largest with 15 species. About management, conservation strategies and climate change 50% species exhibit deciduous nature in the forest. Out etc. In the present study, the Terai region of Uttar Pradesh of total species occurring in the region, about 63% are has been selected for the assessment of tree species as it native to India. -

Tree Resources of Katerniaghat Wildlife Sanctuary, Uttar Pradesh, India with Especial Emphasis on Conservation Status, Phenology and Economic Values
INTERNATIONAL JOURNAL OF ENVIRONMENT Volume-3, Issue-1, Dec-Feb 2013/14 ISSN 2091-2854 Received: 10 January Revised: 17 January Accepted: 21 January TREE RESOURCES OF KATERNIAGHAT WILDLIFE SANCTUARY, UTTAR PRADESH, INDIA WITH ESPECIAL EMPHASIS ON CONSERVATION STATUS, PHENOLOGY AND ECONOMIC VALUES Lal Babu Chaudhary1*, Anoop Kumar2, Ashish K. Mishra3, Nayan Sahu4, Jitendra Pandey5, Soumit K. Behera6 and Omesh Bajpai7 1,2,3,4,6,7Plant Diversity, Systematics and Herbarium Division, CSIR-National Botanical Research Institute, Rana Pratap Marg, Lucknow, Uttar Pradesh-226 001, India 5,7Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, Uttar Pradesh- 221 005, India *Corresponding author: [email protected] Abstract Uttar Pradesh, one of the most populated states of India along international border of Nepal, contributes only about 3% of total forest & tree cover of the country as the major parts of the area is covered by agriculture lands and human populations. The forests are quite fragmented and facing severe anthropogenic pressure in many parts. To protect the existing biodiversity, several forest covers have been declared as National Parks and Wildlife Sanctuaries. In the present study, Katerniaghat Wildlife Sanctuary (KWS) has been selected to assess tree diversity, their phenology and economic values as the trees are the major constituent of any forest and more fascinating among all plant groups. The sanctuary consists of tropical moist deciduous type of vegetation and situated along the Indo-Nepal boarder in Bahraich district of Uttar Pradesh, India. After, thorough assessment of the area, a list of 141 tree species belonging to 101 genera and 38 families have been prepared. -

Central Campus Tree Walk (PDF)
The fast-growing, deciduous Native to eastern and central Sweetgum is native to the US, China, this large, moderate Mexico and as far south as to rapidly growing tree has Nicaragua. The simple leaves a broad, round appearance. are glossy-green, with 5 deep It can grow up to 60 ft tall lobes resembling a star shape. and equally wide. Its foliage Central Campus In fall, the foliage turns red consists of simple, alternately and purple, even without cold arranged leaves of dark-green temperatures. The tree is color, which can be lobed or Tree Walk regarded for its ability to fix ovate. Though most cultivars nitrogen in the soil, provide are fruitless, the fruit is edible shade and resist insect attacks. and is used in jams and pies. Liquidambar styraciflua: Sweetgum 12 16 Morus alba: White Mulberry The deciduous Crape Myrtle, Native to Southeast Asia, the native to India, Southeast Asia, Dwarf Orchid is deciduous and northern Australia and parts grows 6-8 ft tall. The leaves of Oceania, grows 10-20 ft in are 4” long, broad & bi-lobed, height. The alternate, elliptical resembling the hoof of a cow. to rounded leaves are up to The large, white, fragrant 2.75” long. The young leaves flowers, appear at the edges of in spring are reddish and the the branches, have 5 petals, 10 smooth bark comes off in big yellow-tipped stamens, and a green stigma. Traditional flakes. Its crimpy petals are Oviatt Library intense pink to red and bloom medicine used the bark, from mid-summer to early fall. -

1. Tribe CERCIDEAE 1. CERCIS Linnaeus, Sp. Pl. 1: 374. 1753
1. Tribe CERCIDEAE 紫荆族 zi jing zu Chen Dezhao (陈德昭 Chen Te-chao), Zhang Dianxiang (张奠湘); Kai Larsen, Supee Saksuwan Larsen, Michael A. Vincent Leaves alternate, simple, entire or 2-lobed, sometimes parted to base, divided and 2-foliolate. Flowers usually bisexual, rarely unisexual (polygamous or plants dioecious), slightly or conspicuously zygomorphic. Calyx entire, 5-toothed, spathaceous or val- vately 2–5-lobed. Petals usually (2–)5(or 6), subequal to greatly unequal, free. Perfect stamens 10 or 2–9 reduced to staminodes; anthers dorsifixed, opening lengthwise or by apical pores. Ovary stipe free or adnate to receptacle; ovules 1 to numerous. Legumes flattened or turgid. About five genera and 320–350 species: four genera represented in tropical regions, one in temperate parts of the N Hemisphere; two genera and 52 species (28 endemic, two introduced) in China. The concept of the genus Bauhinia as presented here is adopted in the broadest sense. 1a. Legume narrowly winged along ventral suture; perfect stamens 10; flowers purplish red or pink ..................................... 1. Cercis 1b. Legume without wings; perfect stamens usually 3 or 5, if 10 then flowers white, light yellow, or green ...................... 2. Bauhinia 1. CERCIS Linnaeus, Sp. Pl. 1: 374. 1753. 紫荆属 zi jing shu Chen Dezhao (陈德昭 Chen Te-chao), Zhang Dianxiang (张奠湘); Supee Saksuwan Larsen, Michael A. Vincent Shrubs or trees. Leaves alternate, simple, entire, veins palmate, base cordate to truncate or cuneate, apex acute to attenuate or emarginate; stipules caducous, small, scalelike or membranous. Flowers zygomorphic, bisexual, purplish red, pink, or white, in soli- tary racemes or subumbellate clusters on branches of current year or older branches or trunks, flowering before or as leaves expand; bracts scalelike, often imbricate, aggregated at base of racemes; bracteoles minute or absent. -

Timor Plants
4. The botanical and ethnobotanical background This chapter highlights botanical and ethnobotanical information of relevance to the present study. Reference is made to some of the most relevant botanical earlier work carried out in both East and West Timor. The botanical descriptions by Joachim Metzner (1977) are described in a separate section, as they form the only systematic work done in the area under analysis. Finally, other ethnobotanical accounts resulting from relevant anthropological work in Timor are also listed. 4.1 Three hundred years of botanical work in East Timor Forbes (1989, original publication from 1885) and Cinatti (1950b) give comprehensive lists of all early botanical investigations carried out in both East and West Timor, and a table (4.1) listing those works by year, collector and region is given in appendix 18. Forbes, who described and collected plant specimens in the eastern part of Timor between 1882‐83, published his account in 1885. Forbes’ work was later updated by Cinatti, who was a professional forester. Cinatti, whose passion for botany was triggered by Forbes’ and by Castro’s (1943) works, also did his own plant collections in Timor. Dampier was the first of the amateur and professional botanists who during the 18th and 19th centuries arrived in Timor and collected plants (Dampier 1927, originally published in 1697). Like many others in that early period, Dampier worked only in the western part of the island around Kupang. In the nineteenth century, the famous naturalists Alfred Russel Wallace (1962, original work from 1869) and Henry O. Forbes spent time in East and West Timor. -

In Vitro Antioxidant, Cytotoxic and Membrane Stabilizing Activities of Bauhinia Acuminata L
Bangladesh Pharmaceutical Journal 17(1): 99-101, 2014 In vitro Antioxidant, Cytotoxic and Membrane Stabilizing Activities of Bauhinia acuminata L. Mohammad Firoz Khan1, Rabeya Islam Shilpi1, Ridwan Bin Rashid2 and Mohammad A. Rashid3 1Department of Pharmacy, State University of Bangladesh, Dhaka- 1205, Bangladesh 2Department of Microbiology, University of Dhaka, Dhaka- 1000, Bangladesh 3Department of Pharmaceutical Chemistry, University of Dhaka, Dhaka- 1000, Bangladesh Abstract The crude methanol extract of leaves of Bauhinia acuminata and its Kupchan fractions were screened for antioxidant, cytotoxic and membrane stabilizing activities. Among all partitionates the aqueous soluble fraction of B. acuminata demonstrated the highest antioxidant activity with IC50 value of 7.22±0.200 μg/ml. Moreover, the carbon tetrachloride soluble fraction showed significant cytotoxic activity having LC50 value of 12.13±0.215 μg/ml. On the other hand, in hypotonic solution- and heat- induced conditions, the crude methanol extract inhibited haemolysis of human erythrocyte by 63.94±0.14% and 51.95±0.20%, respectively as compared to 81.97±0.77% and 42.11±0.39% demonstrated by the standard acetyl salicylic acid. Key words: Bauhinia acuminata, antioxidant, cytotoxic and membrane stabilizing. Introduction Materials and Methods Bauhinia acuminata L. (Common Name- Dwarf Plant materials: The leaves of B. acuminata were White Bauhinia, Family- Fabaceae) is a species of collected from Khulna and a voucher specimen (DUSH- flowering shrub native to tropical southeastern Asia. The 10775) for this plant sample has been deposited in the bark, flower and root of the B. acuminata are used for Department of Botany, University of Dhaka for future various skin diseases, worms, tumours and diabetes reference. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Comparative Pharmacognostical Evaluation And HPTLC Analysis Of Three Different Species Of Bauhinia Leaves. Abhishek Gupta 1, 3, Jyotsana Dwivedi1, Saba Irshad1, Shikhar Verma2, Siddharth Pragyadeep1, Harinath Dwivedi3, and AKS rawat*1. 1Pharmacognosy & Ethnopharmacology Division, CSIR-National Botanical Research Institute, Lucknow, India 2Amity Institute of Pharmacy, Amity University, Lucknow 3School of Pharmacy, Babu Banarsi Das University, Lucknow, India ABSTRACT Bauhinia purpurea L., Bauhinia variegata L., and Bauhinia acuminata L., commonly known as Kanchanar and mountain ebony belongs to family Caesalpiniaceae. The current study was carried out to provide comparative macro-microscopy, physicochemical parameters and HPTLC analysis of three different Bauhinia species. TS of leaf show different outline, variation in the size of ground tissue region as well as size of xylem vessels of three studied species of Bauhinia. The lower cortex region of B. acuminata is wide (6-7 layered) when compared to other species which are 3-4 layered. Bauhinia acuminata L. have large number of simple trichomes on lower surface while B. variegata L. have few trichome and trichomes are absent in B. purpurea L. The trichome size is long in B. acuminata L. when compared to B. variegata L. Quantitative microscopy revealed that B. purpurea L. and B. acuminata L. have paracytic stomata while B. variegata have anisocytic type of stomata. Stomatal index was found to be highest in lower side of B. purpurea L. leaf. Stomatal number is highest in lower side of B. purpurea L. leaf. Vein islet number and palisade ratio is highest in B.