2019 Moose Progress Research Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Columbia School Consolidation from the Perspective of the Prince George Region
CONTEXTUALIZING CONSOLIDATION: BRITISH COLUMBIA SCHOOL CONSOLIDATION FROM THE PERSPECTIVE OF THE PRINCE GEORGE REGION by THEODORE D. RENQUIST B. A., Simon Fraser University, 1974 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in THE FACULTY OF GRADUATE STUDIES Department of Educational Studies We accept this thesis as conforming to tWe^eajlired standard THE^UNIVERSITY OF BR/'TIS H COLUMBIA December, 1994 ©Theodore Renquist, 1994 In presenting this thesis in partial fulfillment of the requirements for an advanced degree at The University of British Columbia., I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Educational Studies The University of British Columbia 2075 Wesbrook Place Vancouver, Canada V6T 1W5 Date: August 1994 Abstract Throughout the first half of this century controversy surrounded the division of governance between provincial and local authorities. In a general sense this thesis examines the centralizing forces of equality of opportunity promoted by the provincial government versus the forces of decentralization found in the principle of local autonomy. Specifically this thesis examines the reasons why the school districts in die central interior of British Columbia, around Prince George, were consolidated with little or no opposition in 1946 following the recommendations of the Cameron Report. This thesis is a case study of the region approximately in the center of the province that was to become School District No. -

Order in Council 1780/1986
COLUMBIA 1780 APPROVED AND ORDERED SEP 24.1986 Lieutenant-Governer EXECUTIVE COUNCIL CHAMBERS, VICTORIA SEP 24.1986 On the recommendation of the undersigned, the Lieutenant-Governor, by and with the advice and consent of the Executive Council, orders that a general election be held in all the electoral districts for the election of members to serve in the Legislative Assembly; AND FURTHER ORDERS THAT Writs of Election be issued on September 24, 1986 in accordance with Section 40 of the Election Act; AND THAT in each electoral district the place for the nomination of candidates for election to membership and service in the Legislative Assembly shall be the office of the Returning Officer; AND THAT A Proclamation to that end be made. ...-- • iti PROVINCI1‘( ,IT/ ECRETARYT(' AND MINISTER OF GOVERNMENT SERVICES PRESIDING MEMBER OF THE E ECUTIVE COUNCIL (This part is for administrative purposes and it not part of the Order.) Authority under which Order is made: Election Act - sec. 33 f )40 Act and section. Other (specify) Statutory authority checked r9Z 4,4fAr 6-A (Signet, typed or printed name of Legal OfOra) ELECTION ACT WRIT OF ELECTION FORM 1 (section 40) ELIZABETH II, by the Grace of God, of the United Kingdom, Canada and Her Other Realms and Territories, QUEEN, Head of the Commonwealth, Defender of the Faith. To the Returning Officer of the Electoral District of Coquitlam-Moody GREETING: We command you that, notice of time and place of election being given, you do cause election to be made, according to law, of a member (or members -

South Fraser Advisor NR.Pdf
NEWS RELEASE For Immediate Release February 11, 2015 KEY SUPPORT FOR TRADES TRAINING NOW AVAILABLE FOR THE SOUTH FRASER REGION The Industry Training Authority introduces local Apprenticeship Advisor to act as an on-the-ground resource for apprentices and employers Surrey, BC – The Industry Training Authority (ITA) has introduced the Apprenticeship Advisor who will act as an on-the-ground resource for apprentices and employers in the South Fraser region. Over 70 local apprentices, employers and industry representatives gathered at the Sheraton Vancouver Guildford Hotel yesterday to meet Dal Dhatt, ITA’s Apprenticeship Advisor for the South Fraser region, along with Gary Herman, ITA CEO. As the new Apprenticeship Advisor for South Delta, Surrey, Langley and White Rock, Dhatt brings over eight years of experience motivating and counseling individuals towards employment. Having joined the Industry Training Authority in 2007, Dhatt previously worked as the Administrator for Youth Initiatives, responsible for overseeing the Accelerated Credit Enrolment in Industry Training (ACE IT) and Secondary School Apprenticeship (SSA) programs. “ITA is necessary to ensure that our children are prepared for the jobs of tomorrow,” said Peter Fassbender, MLA for Surrey-Fleetwood and Minister of Education for BC. “Dal Dhatt will be instrumental in building the knowledge base and awareness of the BC apprenticeship system and will be a key liaison to apprentices and employer sponsors.” “Congratulations to Dal Dhatt on his appointment as Apprenticeship Advisor -

Order in Council 2433/1979
BRITISH COLUMBIA A .4`t = 2433 APPROVED AND ORDERED sp. 14.1979 EXECUTIVE COUNCIL CHAMBERS, MM. DELTA SER 13.1979 AdmkLund0 On the recommendation of the undersigned, the by and with the advice and consent of the Executive Council, orders that Order-in-Council 2402/79 be amended by deleting the words "by Order-in-Council 175/79" in the second last line and substituting the words "by Order-in-Council 1078/79".1 „ormallo ~sm. PROV C SECRETARY AND MINISTER OF GOVERNMENT SERVICES PRESIDING MEMBER OF THE EXECUTIVE COUNCIL (This part u for administrative purposes and is not part of the Order.) Authority under which Order is made: Act and section Provincial _Elect_Lons Act — section 6 Other (specify) . Statutory authority checked by °Yeist } uv 4 - OG (Signature and typed or printed Meer C:7! BRITISH s.712.5.4?( COLUMBIA 1078 APPROVED AND ORDERED Mt —5.1979 - LreTitii:anT overnor EXECUTIVE COUNCIL CHAMBERS, VICTORIA 12R _51979 On the recommendation of the undersigned, the Lieutenant-Governor, by and with the advice and consent of the Executive Council, orders that pursuant to the provisions of the Provincial Elections Act, a Registrar of Voters and Deputy Registrar of Voters be appointed for each of the electoral districts of the province in accordance with the attached Schedules "A" and "8"; AND FURTHER ORDERS THAT pursuant to the provisions of the Constitution Act, the Interpretation Act, the Provincial Elections Act, and all other powers thereunto enabling, the following regulation be made: "The Chief Electoral Officer and the Registrar-General -

Spring 1982 Volume 19, Number 2 Motor Carrier Branch Reorganized
ISSN 0382-2141 PUBLISHED BYTHE BRITISH COLUMBIA MINISTRYOFTRANSPORTATION AND HIGHWAYS SPRING 1982 VOLUME 19, NUMBER 2 MOTOR CARRIER BRANCH REORGANIZED Four new managers were appointed for the Motor Carrier Branch recently in an effort to streamline carrier operations for better service to the motor carrier industry. The four are Meredith Rowden. Manager of Passenger Licences and Safety; Dave Hammell. Manager of Freight Licencing for the Lower Mainland and Vancouver Island; Ken Knott . Manager of Motor Carrier Compliance within the Province; and Don Murray. Manager of Freight Licencing for the Provincial Region. As part of the change. Doug Tyson. Deputy Superintendent becomes responsible for the Office Managers. Manager of Compliance. and Manager. Financial Investigation (Rates Examiner). All four will remain based at the Burnaby Headquarters, 4240 Manor Street. Ministry Profile AL FITCH--SUPERINTENDENT MOTOR CARRIER BRANCH When AI Fitch. Superintendent of the Motor Carrier Branch started out as a junior office boy 35 years ago his pay cheque was $65 a month plus a cost of living bonus of $ I5. His total yearly salary came to $780. The branch at that time came under the Public Utilities Commission and the Superin tendent was Major R. M. Taylor whose staff in cluded an Assistant Superintendent, Rates Exam iner and Assistant, Accountant, Chief Clerk, general office staff and seven Field Inspectors located at Cranbrook, Nelson, Kelowna, Kamloops, Vancouver and Victoria . Today Head quarters are located at 4240 Manor Street. Burnaby, and there are field offices located in Nanaimo, Prince George. Cranbrook, Dawson Creek, Kamloops, Kelowna and Victoria. Al has the distinction of being the youngest Inspector ever appointed at 22 years of age and the youngest Superintendent at 44. -

2019 Moose Factsheet Final Oct 28 2019
FACTSHEET October 2019 Ministry of Forests, Lands, Natural Resource Operations and Rural Development Moose in B.C. Moose are an iconic animal in British Columbia and play a vital role in the well-being of many communities and individuals. First Nations rely on moose for social, ceremonial, and sustenance purposes. Moose also provide sustenance and recreational opportunities to resident and non- resident hunters. Hunting opportunities provide important social opportunities and economic benefits. Ministry staff and partners are continuing to undertake surveys, research, assess moose health and work closely with First Nations and stakeholders to better inform management decisions. In 2018/19, the ministry’s investments in moose projects totaled nearly $2.7 million, and over $3.3 million in moose projects were funded provincially. Projects for moose enhancement include population inventory, habitat designation, habitat enhancement and assessment of species interactions. The ministry’s moose research project started in 2013 and continues to provide important information for moose management. This fact sheet provides an update on implementing the 2015 Provincial Framework for Moose Management, and “A Strategy to Help Restore Moose Populations in British Columbia.” Highlights of the work conducted over the past year include: • Calf survival was confirmed to be an important factor in population trends. Initial findings from this new approach suggest early calf mortality occurs during their first summer and in late winter and early spring. This mortality appears to be the main contributing factor to the population declines. The ministry is investigating multiple factors influencing the high mortality rates and will use the results to better inform management decisions and project investments. -

Gibsons' Council Presses Holly Wreaths Last Saturday
Provincial LIbr « Victoria* B."C. xn j... JUST FINE FOOD A Complete Line DANNY'S of Men's Clothing ' DINING ROOM SERVING THE GROWING SUNSHINE COAST Marine Men's Wear Gibsons — Ph. 886-9815 Lid. Published in Gibsons, B.C. Volume 15, Number 49, December 21, 1961. 7c per copy Ph. 886-2116 — Gibsons. B.C. " ~ —" — -'— — -•• -—,T* . .~-~~ —~.T". ' • •-•——-~^__-_»__»«W«K_« Scouts HOLLY ON SALE The Girl Guides sold all their Gibsons' council presses holly wreaths last Saturday. entertain Next Saturday/ :afternoon,fpep.i 23, they will be selling cut holiy Members of the OAPO; were guests on Friday evening, .Dec. outside Super Valu, as well as a 15 at a concert given by First Christmas tree which has been Y Gibsons -Municipal Cpuncil af- tc infci^ri M_v;Bonrter- council still body representing . both organiz Gibsons Boy Scouts arid their donated. (er discussion Tuesday night on sought an Increased police; force. ed and unorganized areas. If the f leaders. > Mrs. Allen will take orders for a letter from ' Attorney-General A request for the handling of local recreation committee was The guests were transported JJonner replying to a request for recreation . .commission govern-, for Gibsons alone, no..problem to and from the affair, with a Christm'as trees. Phone 886-2324. jnore police for Gibsons area,. ment .grants throughmunicipal would arise. Scout' in attendance with each moved that Mr. Bonner-be in-, clerks was presented council by An attack warning siren has car. formed council had ;riot changed the provincial recreation depart-/ been placed high .up at the south The program was well chosen, its mind and still wanted more ment. -

Report of the Chief Electoral Officer on the 1989 Provincial Enumeration
Report of the Chief Electoral Officer on the 1989 Provincial Enumeration Enumeration ’89 Table of Contents ENUMERATION ‘89 Introduction. ..................1 Registered Voters. ..............3 Expenditures Enumerators’ Expenses by Electoral Disrict . .......4 Registrars’ of Voters Expenses . ..............6 Other Expenses . ................7 Summary of Expenditures . ..............7 Registrars of Voters . .............8 Conclusion . ..................9 Map Introduction Section 17. (1) of the Election Act requires that: “During each Parliament of the Legislature, beginning on the first Monday in May in the third calendar year after the general election for that Parliament, there shall be, unless the Legislature is earlier dissolved, an enumeration of voters in all electoral districts.” A total of 6,607 enumerators were employed during Enumeration ‘89, which commenced on Monday, May 1st. Door-to-door visitations were completed on May 20th. Enumerators visited each residence in the Province in order to obtain completed and signed application cards from new applicants, and where residents were already registered, to confirm those registrations. If prospective voters were not at home at the time of the enumerator’s first visit, a second visit was automatically made. At that time, if they were again not at home, application cards were left for completion and return to the Registrar of Voters. Postage-paid envelopes were included for the convenience of the applicants. Residential visits were made from 9:00 a.m. - 9:00 p.m., Monday to Saturday, and on Sunday from 1:00 p.m. - 6:00 p.m. The revision of voters’ files was completed on July 28th. Phase One of the Provincial enumeration concluded with the mailing of plastic coated Voter Identification Cards to all registered voters. -

Order in Council 1078/1979
-4? osemnse. BRITISH t COLUMBIA 1078 APPROVED AND ORDERED APR. -5.1979 •grz -.1646—, /9Larfa gig!T 414 114 —6'..7./610/P-2/ 0 es. 440-1/111 EXECUTIVE COUNCIL CHAMBERS, VICTORIA Am -51979 'Or44733/1-17;11 Ars./..t., 74 if /04.0/ On the recommendation of the undersigned, the Lieutenant-Governor, by and with the advice and consent wir of the Executive Council, orders that pursuant to the provisions of the Provincial p..7.3,/e/ Elections Act, a Registrar of Voters and Deputy Registrar of Voters be appointed "47:/1:;, for each of the electoral districts of the province in accordance with the //.77 attached Schedules "A" and "B"; //jS771.8 AND FURTHER ORDERS THAT pursuant to the provisions of the Constitution Act, the /2/ .44:/gA Interpretation Act, the Provincial Elections Act, and all ots.er powers thereunto .;v0/12 enabling, the following regulation be made: "The Chief Electoral Officer and the Registrar-Geleral of "19-1/i.a. Voters, the Registrars of Voters, and all other persons appointed to 'rye/z functions, procedures and other duties under the Provincial Elections Act are hereby empowered to perform such of those functions, R",( 81 procedures and duties as are necessary to prepare the list of voters 04CAU for each of the electoral districts constituted under the Constitution 23o8i8X Act Amendment Act, 1978, and without limiting the generality of the 3s//83 3.13a3 foregoing, the appropriate Registrars of Voters are hereby authorized: (1) To perform the functions set out in Section 23A of the 34t Provincial Elections Act. -
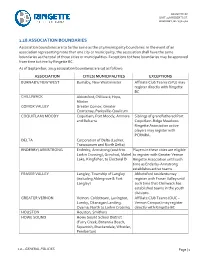
1.18 ASSOCIATION BOUNDARIES Association Boundaries Are to Be the Same As the City/Municipality Boundaries
RINGETTE BC UNIT 258 ROBERTS ST. BURNABY, BC V5G 4G2 1.18 ASSOCIATION BOUNDARIES Association boundaries are to be the same as the city/municipality boundaries. In the event of an association representing more than one city or municipality, the association shall have the same boundaries as the total of those cities or municipalities. Exceptions to these boundaries may be approved from time to time by Ringette BC. As of September, 2019 association boundaries are set as follows: ASSOCIATION CITIES/ MUNICIPALITIES EXCEPTIONS BURNABY/ NEW WEST Burnaby, New Westminster Affiliate Club Teams (SFU) may register directly with Ringette BC CHILLIWACK Abbotsford, Chilliwack, Hope, Mission COMOX VALLEY Greater Comox, Greater Courtenay, Parksville-Qualicum COQUITLAM/ MOODY Coquitlam, Port Moody, Anmore Siblings of grandfathered Port and Belcarra Coquitlam-Ridge Meadows Ringette Association active players may register with PCRMRA. DELTA Corporation of Delta (Ladner, Tsawwassen and North Delta) ENDERBY/ ARMSTRONG Enderby, Armstrong (south to Players in these cities are eligible Larkin Crossing), Grindrod, Mabel to register with Greater Vernon Lake, Kingfisher, to Electoral D Ringette Association until such time as Enderby-Armstrong establishes active teams. FRASER VALLEY Langley, Township of Langley Abbotsford residents may (including Aldergrove & Fort register with Fraser Valley until Langley) such time that Chilliwack has established teams in the youth divisions. GREATER VERNON Vernon, Coldstream, Lavington, Affiliate Club Teams (OUC – Lumby, Okanagan Landing, Vernon Campus) may register Oyama, North to Larkin Crossing directly with Ringette BC HOUSTON Houston, Smithers HOWE SOUND Howe Sound School District (Furry Creek, Britannia Beach, Squamish, Brackendale, Whistler, Pemberton) 1.0 – GENERAL POLICIES Page | 1 RINGETTE BC UNIT 258 ROBERTS ST. -

2018 Moose Fact Sheet
FACT SHEET October 2018 Ministry of Forests, Lands, Natural Resource Operations and Rural Development Moose in B.C. The moose is an iconic animal. First Nations rely on moose for social, ceremonial, and sustenance purposes. Moose also provide sustenance and recreational opportunities to resident and non- resident hunters. Hunting opportunities provide economic benefits through the sale of hunting licences and associated travel and accommodation expenditures. Collectively, moose play a vital role in the well-being of many communities and individuals. Ministry staff and partners are continuing research, undertaking surveys, assessing moose health and working closely with First Nations and stakeholders to gain more understanding and better inform management decisions. In 2018/19, the ministry’s investments in moose projects totaled nearly $2.05 million. The ministry’s five-year moose research project, started in 2013, was recently extended for another five years and expanded to investigate causes of calf mortality. This fact sheet also provides an update on implementing the 2015 Provincial Framework for Moose Management, and “A Strategy to Help Restore Moose Populations in British Columbia.” Highlights of the work conducted over the past year include: • Calf survival was identified as an important factor in population trends. Initial findings suggest calf mortality in late winter and spring are a main contributing factor to the declines. The ministry is investigating multiple factors that may be causing the high mortality rates observed as part of the research project, and will use the results to better inform management decisions and project investments. • The 2017 Plateau fires in the Chilcotin were aerially surveyed and show ungulates remaining in burned landscapes. -

Term 020133 (September to December, 2013)
Term 020133 (September to December, 2013) PREVIOUS CONTINUING STUDIES HISTORY BY GENERAL AREA OF RESIDENCE NO YES Vancouver 1213 39.2% 1878 60.8% Richmond 272 43.7% 351 56.3% Burnaby 188 46.0% 221 54.0% North Vancouver 68 56.2% 53 43.8% West Vancouver 16 32.0% 34 68.0% New Westminster 35 47.9% 38 52.1% Surrey 138 52.3% 126 47.7% Delta 42 45.7% 50 54.3% White Rock 8 53.3% 7 46.7% Pt. Moody/Coquitlam/PoCo 80 49.1% 83 50.9% Pitt Meadows/Maple Ridge 15 57.7% 11 42.3% Langley 18 58.1% 13 41.9% Mission/Abbotsford/Chilliwack 30 73.2% 11 26.8% Vancouver Island 11 64.7% 6 35.3% Other B.C. 24 60.0% 16 40.0% Other Provinces 17 34.0% 33 66.0% Unknown 50 61.7% 31 38.3% GENERAL BREAKDOWN OF WHERE LANGARA COLLEGE CS STUDENTS LIVE Median Course Mean Course Count Percent Load Load Vancouver 3091 59.6% 1 1.5 Richmond 623 12.0% 1 1.8 Burnaby 409 7.9% 1 1.8 North Vancouver 121 2.3% 1 1.8 West Vancouver 50 1.0% 1 1.7 New Westminster 73 1.4% 1 1.9 Surrey 264 5.1% 1 2.0 Delta 92 1.8% 1 1.8 White Rock 15 .3% 2 2.1 Pt. Moody/Coquitlam/PoCo 163 3.1% 1 2.0 Pitt Meadows/Maple Ridge 26 .5% 2 2.5 Langley 31 .6% 1 1.9 Mission/Abbotsford/Chilliwack 41 .8% 1 2.7 Vancouver Island 17 .3% 1 2.5 Other B.C.