Intracellular Mechanisms of A-Trichothecenes Involved in the Regulation of Cell Survival and Apoptosis: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: a Systematic Review
molecules Review Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review Magdalena Polak-Sliwi´ ´nska* and Beata Paszczyk Faculty of Food Sciences, University of Warmia and Mazury in Olsztyn, Plac Cieszy´nski1, 10-726 Olsztyn, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-89-523-45-84 Abstract: Trichothecene mycotoxins are sesquiterpenoid compounds primarily produced by fungi in taxonomical genera such as Fusarium, Myrothecium, Stachybotrys, Trichothecium, and others, under specific climatic conditions on a worldwide basis. Fusarium mold is a major plant pathogen and produces a number of trichothecene mycotoxins including deoxynivalenol (or vomitoxin), nivalenol, diacetoxyscirpenol, and T-2 toxin, HT-2 toxin. Monogastrics are sensitive to vomitoxin, while poultry and ruminants appear to be less sensitive to some trichothecenes through microbial metabolism of trichothecenes in the gastrointestinal tract. Trichothecene mycotoxins occur worldwide however both total concentrations and the particular mix of toxins present vary with environmental condi- tions. Proper agricultural practices such as avoiding late harvests, removing overwintered stubble from fields, and avoiding a corn/wheat rotation that favors Fusarium growth in residue can reduce trichothecene contamination of grains. Due to the vague nature of toxic effects attributed to low concentrations of trichothecenes, a solid link between low level exposure and a specific trichothecene is difficult to establish. Multiple factors, such as nutrition, management, and environmental con- ditions impact animal health and need to be evaluated with the knowledge of the mycotoxin and concentrations known to cause adverse health effects. Future research evaluating the impact of low-level exposure on livestock may clarify the potential impact on immunity. -

In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity
CORE Metadata, citation and similar papers at core.ac.uk Provided by Ghent University Academic Bibliography Article In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity Sandra Debevere 1,2, An Cools 3, Siegrid De Baere 1, Geert Haesaert 4, Michael Rychlik 5, Siska Croubels 1,† and Veerle Fievez 2,*,† 1 Department of Pharmacology, Toxicology and Biochemistry, Faculty of Veterinary Medicine, Ghent University, Salisburylaan 133, 9820 Merelbeke, Belgium; [email protected] (S.D.); [email protected] (S.D.B.); [email protected] (S.C.) 2 Department of Animal Sciences and Aquatic Ecology, Faculty of Bioscience Engineering, Ghent University, Coupure links 653, 9000 Ghent, Belgium 3 Department of Nutrition, Genetics and Ethology, Faculty of Veterinary Medicine, Ghent University, Heidestraat 19, 9820 Merelbeke, Belgium; [email protected] 4 Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Valentin Vaerwyckweg 1, 9000 Ghent, Belgium; [email protected] 5 Chair of Analytical Food Chemistry, Technical University of Munich, Maximus-von-Imhof-Forum 2, 85354 Freising, Germany; [email protected] * Correspondence: [email protected]; Tel.: +32-9-264-9002 † Shared last author. Received: 3 January 2020; Accepted: 31 January 2020; Published: 5 February 2020 Abstract: Ruminants are generally considered to be less susceptible to the effects of mycotoxins than monogastric animals as the rumen microbiota are capable of detoxifying some of these toxins. Despite this potential degradation, mycotoxin-associated subclinical health problems are seen in dairy cows. In this research, the disappearance of several mycotoxins was determined in an in vitro rumen model and the effect of realistic concentrations of those mycotoxins on fermentation was assessed by volatile fatty acid production. -

Vomitoxin: Natural Occurrence on Cereal Grains and Significance As a Refusal and Emetic Factor to Swine
Vomitoxin: Natural Occurrence on Cereal Grains and Significance as a Refusal and Emetic Factor to Swine R. F. Vesonder B.S. and C. W. Hesseltine Ph.D.* Fifty 12,13-epoxytrichothecenes have been reported in the literature; of these diacetoxyscirpenol, T-2 toxin, nivalenol and vomitoxin have been naturally occurring in cereal grains throughout the world. Vomitoxin is produced in ears of corn prior to harvest when wet, cool weather precedes which favours Gibberella zeae growth. This type of infected corn, which often contains vomitoxin, is associated with the refusal and emetic syndrome exhibited by swine. Since 1916, emesis in humans resulting from grains is indicated by growth of the mould Fusaria in the field is meagre. The ability ingestion of food prepared from Fusarium G. zeae (perfect stage of F. graminearuml of G. zeae to render grains unwholesome for infected cereal grains has been recorded in on the kernels and flowering head. On corn, consumption by swine and other farm several areas around the world. In the Soviet G. zeae occurs as a reddish mould that animals may be dependent on the type of Union, .it was known as the drunken bread begins as observable growth on the ear tip. hybrid, but studies of whether G. zeae intoxication. Sporadic food poisoning featur This condition is referred to as Gibberella or infection can be controlled by breeding 8 ing vomiting, nausea, somnolence, headache pink ear rot • new plants have not been conducted. and convulsion occurred in Japan during Outbreaks of barley scabbed with Emetic substances were extracted from the 1950's and the late 1940's. -

Mold, Fungi and Mycotoxins Technical Note
Technical Note Mold, Fungi and Mycotoxins Patricia L. Atkins • Senior Applications Scientist and Alan H. Katz, PhD • Director, Global Product Manager - Chemicals Mold and fungi are ubiquitous in the world. Their size and the mobility of their spores cause rapid spread through agricultural products under favorable environmental conditions. Significant crop losses and foodborne illness can be attributed to mold and fungi when secondary metabolites, called mycotoxins, develop. The botanicals, cannabis and food industries battle continuously with such contaminants. In the United States, the CDC estimates that 48 million people get sick from foodborne illnesses, and up to 3,000 die from foodborne diseases each year. More than 250 agents are known to cause foodborne illness and are introduced through contamination, improper handling practices and sanitation. These agents can be chemical, physical or biological. Biological contaminants in the form of microbes are by far one of the greatest concerns for illness. The five types of microbes are bacteria, viruses, parasites, protozoa, and fungi. Fungi are a very diverse kingdom of single and multicellular organisms, (they were once considered plants). We now know that fungi are more closely related genetically to animals than plants. Fungi (which are nonphotosynthetic) derive their nutrients from decaying or dead matter (saprophytes), or from living organisms (parasites). Scientists have identified a hundred thousand known species of fungi. This is a fraction of the over 1 million likely species present on Earth. The classification of Kingdom Fungi is constantly being debated with the influx of DNA data. Currently the kingdom contains seven phyla which span the different forms of fungal organisms from single-celled yeasts to multicellular mushrooms. -

Mould Growth on Building Materials Secondary Matabolites, Mycoxocins and Biomarkers
Downloaded from orbit.dtu.dk on: Oct 05, 2021 Mould growth on building materials Secondary matabolites, mycoxocins and biomarkers Nielsen, Kristian Fog Publication date: 2001 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Nielsen, K. F. (2001). Mould growth on building materials: Secondary matabolites, mycoxocins and biomarkers. General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Mould growth on building materials Secondary metabolites, mycotoxins and biomarkers Kristian Fog Nielsen The Mycology Group Biocentrum-DTU Technical University of Denmark Lyngby 2002 Mould growth on building materials Secondary metabolites, mycotoxins and biomarkers ISBN 87-88584-65-8 © Kristian Fog Nielsen [email protected] Phone + 45 4525 2600 Fax. + 45 4588 4922. The Mycology Group, Biocentrum-DTU Technical University of Denmark, Building 221 Søltofts Plads, Building 221, DK-2800 Kgs. Lyngby, Denmark Energy and Indoor Climate Division Danish Building Research Institute Dr. -

Ovine Ill-Thrir in Nova Scotia. 13. Anorexia and Digestibility Decline in Female Lambs Given 3,7,1 Ph -3-Acetoxy-7,15-Dihydroxy
PROC. N.S. INST. SCI. (19961 Volume 41 , Part 1 & 2, pp. 39-47. OVINE ILL-THRIR IN NOVA SCOTIA. 13. ANOREXIA AND DIGESTIBILITY DECLINE IN FEMALE LAMBS GIVEN 3,7,1 PH3-3-ACETOXY-7,15-DIHYDROXY-12, 3-EPOXYTRICHOTHEC-9-EN-8-0NE' D. BREWER, A.J. MCALEES' and A. TAYLOR' National Research Council of Canada, Institute of Marine Biosciences, Halifax, Nova Scotia B3H 3Z1 . l H (I. 3.7,1' l-J-Acetoxy-7, 15-dihydroxy-12, 13-epoxytrichothec-9-en-8-one R=H , R'",Ad was 1 produced by Fusarium culmorum (CMI14764) grown on a defined medium supplemented with 5. H2- mevalonic acid. The metabolite was rigOfously purified and the position of the 3·1H label unequivocally determined by Iritylation of the 15-hydroxy group. hydrolysis of the ester and regiospecific oxidation of the 3-hydroxy group thus generated. The metabolite (I, R.H, R'aAc) given as a single intraruminal dose (5 mg kg-I) to female lambs resulted in a 44% decline in food intake and a S%decrease in apparent digestibility of this feed , in the 4 days follOWing administration of the toxin (I, R-H, R',.,Ac). Introduction Deoxynivalenol (=vomitoxin = Rdtoxin = 3,7,1 5-trihydroxy-12, 13-epoxytrichothec- 9-en-8-one = I, R=R'=H, Yoshizawa and Morooka, 1973) was shown to be an emetic when present in the diet of pigs (Vesonder et aI., 1973) and subsequently proved to induce anorexia in mice (Pestka et aI., 1986), swine (Friend et aI., 1986, Prelusky, 1994) and sheep (Harvey et al., 1986). -

Children and Food Safety
TRAINING FOR THE HEALTH SECTOR [Date …Place …Event …Sponsor …Organizer] CHILDREN AND FOOD SAFETY Children's Health and the Environment WHO Training Package for the Health Sector World Health Organization www.who.int/ceh 1 <<NOTE TO USER: Please add details of the date, time, place and sponsorship of the meeting for which you are using this presentation in the space indicated.>> <<NOTE TO USER: This is a large set of slides from which the presenter should select the most relevant ones to use in a specific presentation. These slides cover many facets of the problem. Present only those slides that apply most directly to the local situation in the region.>> This presentation provides some of the basic information needed to understand how food contamination affects children. It stresses the ways children from preconception through adolescence are different from adults in their exposure to food contaminants. Children and Food Safety LEARNING OBJECTIVES After this presentation, individuals will understand: Major foodborne risks for Embryo / foetus Breast and bottle-fed infants Children and infants receiving complementary foods How to reduce food contamination during Production Storage Preparation 2 <<READ SLIDE>> Children and Food Safety FOODBORNE DISEASES HOW LARGE IS THE PROBLEM? Only estimates are available Reporting varies according to the source 1.5 billion cases diarrhoea annually (excluding China) 30-70% are food-related 1.8 million deaths mostly in children < 5 years Most of morbidity affects children Vicious circle of diarrhoea and malnutrition 3 <<NOTE TO USER: INSERT LOCAL/NATIONAL/REGIONAL ESTIMATES>> Definition of foodborne diseases : Foodborne diseases are defined as diseases, usually either infectious or toxic in nature, caused by agents that enter the body through the ingestion of food. -

Fusarium Graminearum and Its Trichothecene Chemotype Diversity
THE CONTINUING BATTLE BETWEEN WHEAT AND FUSARIUM GRAMINEARUM: UNDERSTANDING THE MOLECULAR PHYLOGENETIC RELATIONSHIPS, CHEMOTYPE DIVERSITY AND TRICHOTHECENE BIOSYNTHESIS GENE EXPRESSION PATTERNS By CHAMI CHATHURANGI AMARASINGHE A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Department of Plant Science University of Manitoba Winnipeg, Manitoba Canada Copyright ©2016 by CHAMI CHATHURANGI AMARASINGHE ABSTRACT Fusarium head blight (FHB) continues to threaten the economic sustainability of wheat and barley production in Canada and worldwide. The overall goal of this thesis is to expand our current knowledge of the FHB pathogen, Fusarium graminearum and its trichothecene chemotype diversity. Continuous monitoring of trichothecene chemotypes may well inform on the potential risk and the type of Fusarium populations present in a given region. Fusarium populations in Winnipeg and Carman, Manitoba were examined using chemotype as a marker in the field. Rapid expansion of the 3-acetyldeoxynivalenol (3-ADON) chemotype was observed in Winnipeg and Carman. 3-ADON chemotype is consistently found at high frequencies over the previously common 15-acetyldeoxynivalenol (15-ADON) chemotype, suggesting that the shift in pathogen populations is continuing. This study provides the first evidence on the presence of nivalenol (NIV) producing F. cerealis strains in winter wheat in Manitoba, Canada. Therefore, discovery of NIV producing F. cerealis in wheat poses a serious concern for the wheat industry in Canada. Phylogenetic, chemotypic, phenotypic, and pathogenic abilities of 150 strains of F. graminearum species complex (FGSC) from eight countries were investigated. Type and amount of trichothecenes produced by a strain are key factors in determining the level of aggressiveness of that strain regardless of its species origin. -

Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed
Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Edited by Ting Zhou Printed Edition of the Special Issue Published in Toxins www.mdpi.com/journal/toxins Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Promising Detoxification Strategies to Mitigate Mycotoxins in Food and Feed Special Issue Editor Ting Zhou MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Ting Zhou Guelph Research and Development Center (AAFC) Canada Editorial Office MDPI St. Alban-Anlage 66 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Toxins (ISSN 2072-6651) from 2016 to 2018 (available at: http://www.mdpi.com/journal/toxins/special issues/promising detoxification) For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03897-027-9 (Pbk) ISBN 978-3-03897-028-6 (PDF) Cover image courtesy of Yousef I. Hassan. Articles in this volume are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book taken as a whole is c 2018 MDPI, Basel, Switzerland, distributed under the terms and conditions of the Creative Commons license CC BY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0/). -
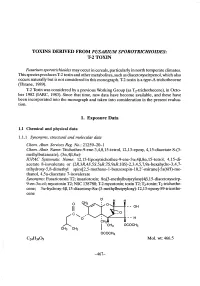
T-2 Toxin. Ten Rats Were Given Nicotinamide Only, and Another 10 Rats Served As Untreated Controls
TOXINS DERID FROM FUSARIUM SPOROTRICHIOIDES: T-2 TOXIN Fusarium sporotrichioides may occur in cereals, particularly in north temperate climates. This species produces T-2 toxin and other metabolites, such as diacetoxyscirpenol, which also occurs naturally but is not considered in this monograph. T-2 toxin is a tye-A trichothecene (Thrane, 1989). T-2 Toxin was considered by a previous Working Group (as T2-trichothecene), in Octo- ber 1982 (IARC, 1983). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evalua- tion. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Chem. Abstr. Services Reg. No.: 21259-20-1 Chem. Abstr. Name: Trichothec-9-ene-3,4,8,15-tetrol, 12,13-epoxy, 4,15-diacetate 8-(3- methylbutanoate), (3æ,4ß,8æ)- IUPAC Systematic Name: 12,13-Epoxyrichothec-9-ene-3æ,4ß,8æ,15-tetrol, 4,15-di- acetate 8-isovalerate or (2R,3R,4S,5S,5aR,7S,9aR,10S)-2,3,4,5,7,9a-hexahydro-3,4,7- trihydroxy- 5 ,8-dimethyl spiro(2,5- methano-1- benzoxepin-1 0,2' -oxirane)- 5a( 6H)-me- thanol, 4,5a-diacetate 7-isovalerate Synonyms: Fusariotoxin TI; insariotoxin; 8æ(3-methylbutyloxy)4ß,15-diacetoxyscirp- 9-en-3æ-ol; mycotoxin TI; NSC 138780; T-2 mycotoxin; toxin TI; T2-toxin; T2-trichothe- cene; 3æ-hydroxy-4ß, 15-diacetoxy-8æ-(3-methylbutyloxy)-12, 13-epoxy-89-tricothe- cene I; H H o - - OH Il - - H OCOCH3 CH3rc'-o... CH3 OCOCH3 Ci.3409 MoL. -

Fate of Deoxynivalenol During Wet Milling
FATE OF DEOXYNIVALENOL DURING WET MILLING A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Ana María Magallanes López In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Program: Cereal Science September 2018 Fargo, North Dakota North Dakota State University Graduate School Title FATE OF DEOXYNIVALENOL DURING WET MILLING By Ana María Magallanes López The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of MASTER OF SCIENCE SUPERVISORY COMMITTEE: Dr. Senay Simsek Chair Dr. Frank A. Manthey Dr. Linda Dykes Dr. Saleem Shaik Approved: 09/07/18 Dr. Richard Horsley Date Department Chair ABSTRACT The fungal disease Fusarium head blight affects cereal grains and can produce mycotoxins, like the water-soluble deoxynivalenol (DON). Wheat wet milling process begins with ground endosperm obtained by dry milling and ends with the separation of starch from gluten. Research was conducted on hard red spring wheat and durum wheat samples naturally contaminated with DON. The fate of DON in wheat dry milled fractionations (farina/semolina, shorts, and bran) during wet milling was investigated. Three wet milling processes were evaluated. DON levels were assessed by GC-ECD. Results showed that DON was present in all dry milled fractions. DON concentration in farina and semolina exceeded the safety threshold for human consumption. After wet milling farina and semolina, nearly all the DON was found in the water-soluble fraction, regardless the wet milling process. A negligible level of DON was found in the gluten extracted from HRSW with Martin wet milling process. -

Open Zitomer.Pdf
The Pennsylvania State University The Graduate School College of Agricultural Sciences MYCOTOXICOLOGY TO ACCOMPANY PHYLOGENETIC REVISIONS TO THE GENUS FUSARIUM A Thesis in Plant Pathology by Nicholas C. Zitomer © 2006 Nicholas C. Zitomer Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2006 The thesis of Nicholas C. Zitomer was reviewed and approved* by the following: Gretchen A. Kuldau Assistant Professor of Plant Pathology Thesis Co-Adviser Co-Chair of Committee David M. Geiser Associate Professor of Plant Pathology Thesis Co-Adviser Co-Chair of Committee Erick D. DeWolf Assistant Professor of Plant Pathology Douglas D. Archibald Research Associate, College of Agricultural Sciences A. Daniel Jones Senior Scientist, Department of Chemistry Barbara J. Christ Professor of Plant Pathology Head of the Department of Plant Pathology *Signatures are on file in The Graduate School. iii ABSTRACT Fusarium species traditionally have been and still are problematic to identify using morphology. This is an issue of concern since many fusaria are toxigenic, producing such toxins as trichothecenes, fumonisins and zearalenone. Fumonisins are sphingolipid analogues associated primarily with the Gibberella fujikuroi species complex (GFC). Fusarium trichothecenes are sesquiterpenoid mycotoxins, and are generally divided into two categories: type A, which lack oxygen at the C-8 position and include T-2 toxin and diacetoxyscirpenol, and type B, which include nivalenol and deoxynivalenol. Zearalenone is an estrogenic mycotoxin. Phylogenetically characterized isolates were subjected to mycotoxin analysis via high performance liquid chromatography and atmospheric pressure chemical ionization mass spectrometry (HPLC/APCI-MS), HPLC and electrospray ionization mass spectrometry (HPLC/ESI-MS), or HPLC using fluorescence detection.