Apocrine Carcinoma of the Breast: a Comprehensive Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Estrogen Receptor Isoforms and Variants in Breast Cancer Cell Lines
EXPERIMENTAL AND THERAPEUTIC MeDICINE 2: 537-544, 2011 Analysis of estrogen receptor isoforms and variants in breast cancer cell lines MAIE AL-BADER1, CHRISTOPHER FORD2, BUSHRA AL-AYADHY3 and ISSAM FRANCIS3 Departments of 1Physiology, 2Surgery, and 3Pathology, Faculty of Medicine, Kuwait University, Safat 13110, Kuwait Received November 22, 2010; Accepted February 14, 2011 DOI: 10.3892/etm.2011.226 Abstract. In the present study, the expression of estrogen domain C, the DNA binding domain; domains D/E, bearing receptor (ER)α and ERβ isoforms in ER-positive (MCF7, both the activation function-2 (AF-2) and the ligand binding T-47D and ZR-75-1) and ER-negative (MDA-MB-231, SK-BR-3, domains; and finally, domain F, the C-terminal domain (6,7). MDA-MB-453 and HCC1954) breast cancer cell lines was The actions of estrogens are mediated by binding to ERs investigated. ERα mRNA was expressed in ER-positive and (ERα and/or ERβ). These receptors, which are co-expressed some ER-negative cell lines. ERα ∆3, ∆5 and ∆7 spliced in a number of tissues, form functional homodimers or variants were present in MCF7 and T-47D cells; ERα ∆5 heterodimers. When bound to estrogens as homodimers, the and ∆7 spliced variants were detected in ZR-75-1 cells. transcription of target genes is activated (8,9), while as heterodi- MDA-MB-231 and HCC1954 cells expressed ERα ∆5 and ∆7 mers, ERβ exhibits an inhibitory action on ERα-mediated gene spliced variants. The ERβ1 variant was expressed in all of the expression and, in many instances, opposes the actions of ERα cell lines and the ERβ2 variant in all of the ER-positive and (7,9). -

Neprilysin Is Required for Angiotensin-(1-7)
Page 1 of 39 Diabetes NEPRILYSIN IS REQUIRED FOR ANGIOTENSIN-(1-7)’S ABILITY TO ENHANCE INSULIN SECRETION VIA ITS PROTEOLYTIC ACTIVITY TO GENERATE ANGIOTENSIN-(1-2) Gurkirat S. Brara, Breanne M. Barrowa, Matthew Watsonb, Ryan Griesbachc, Edwina Chounga, Andrew Welchc, Bela Ruzsicskad, Daniel P. Raleighb, Sakeneh Zraikaa,c aVeterans Affairs Puget Sound Health Care System, Seattle, WA 98108, United States bDepartment of Chemistry, Stony Brook University, Stony Brook, NY 11794, United States cDivision of Metabolism, Endocrinology and Nutrition, Department of Medicine, University of Washington, Seattle, WA 98195, United States dInstitute for Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY 11794, United States Short Title: Angiotensin-(1-7) and insulin secretion Word count: 3997; Figure count: 8 main (plus 3 Online Suppl.); Table count: 1 Online Suppl. Correspondence to: Sakeneh Zraika, PhD 1660 South Columbian Way (151) Seattle, WA, United States Tel: 206-768-5391 / Fax: 206-764-2164 Email: [email protected] 1 Diabetes Publish Ahead of Print, published online May 30, 2017 Diabetes Page 2 of 39 ABSTRACT Recent work has renewed interest in therapies targeting the renin-angiotensin system (RAS) to improve β-cell function in type 2 diabetes. Studies show that generation of angiotensin-(1-7) by angiotensin converting enzyme 2 (ACE2) and its binding to the Mas receptor (MasR) improves glucose homeostasis, partly by enhancing glucose-stimulated insulin secretion (GSIS). Thus, islet ACE2 upregulation is viewed as a desirable therapeutic goal. Here, we show that although endogenous islet ACE2 expression is sparse, its inhibition abrogates angiotensin-(1-7)-mediated GSIS. However, a more widely expressed islet peptidase, neprilysin, degrades angiotensin-(1-7) into several peptides. -

Expression of Estrogen Receptor-Beta Isoforms in Barrett's
ANTICANCER RESEARCH 24: 2919-2924 (2004) Expression of Estrogen Receptor-Beta Isoforms in Barrett’s Metaplasia, Dysplasia and Esophageal Adenocarcinoma LIANG LIU, MINNI CHIRALA and MAMOUN YOUNES Departments of Pathology, Baylor College of Medicine and The Methodist Hospital, Houston, TX 77030, U.S.A. Abstract. We have previously shown that the majority of and beta (ER-B). ER-A is mainly expressed in female sex esophageal adenocarcinomas (EA), and its precursor Barrett’s organs, such as breast and uterus (2), whereas ER-B is metaplasia (BM), express estrogen receptor beta (ER-B). Several expressed in both sex organs and other tissues, such as isoforms of ER-B have been described and are presumed to have prostate, lung, thyroid, adrenal cortex and testis (3). Several different functions, but their distribution in BM and EA is not ER-B isoforms have been identified and characterized in known. The aim of this work was to determine which ER-B many non-gynecologic tumors including carcinomas of the isoforms are expressed in EA and BM. Sections of formalin-fixed lung (4), prostate (5), colon (6, 7) and stomach (8). and paraffin-embedded esophageal tissue from 33 esophageactomy Tamoxifen, a specific ER-B antagonist, has been successfully specimens, of which 27 had invasive EA, were stained for the ER- used in the treatment of patients with breast carcinoma B isoforms ER-B1, ER-B2, ER-B3 and ER-B5 utilizing the (9,10) and it has been shown that ER-B status is a significant immunoperoxidase method. ER-B1 was detected in 23 out of 27 predictor of survival in women with breast carcinoma treated (85%) EA compared to 3 out of 14 (21%) Barrett’s metaplasia with mastectomy and adjuvant tamoxifen (11). -

View a Copy of This Licence, Visit
Choi et al. BMC Cardiovascular Disorders (2020) 20:360 https://doi.org/10.1186/s12872-020-01636-5 RESEARCH ARTICLE Open Access Soluble neprilysin and long-term clinical outcomes in patients with coronary artery disease undergoing percutaneous coronary intervention: a retrospective cohort study Ik Jun Choi1, Sungmin Lim2* , Youngdeok Hwang3, Dongjae Lee1, Won Jik Lee1, Kwan Yong Lee1, Mi-Jeong Kim1 and Doo Soo Jeon1 Abstract Background: Neprilysin has an essential role in regulating fluid balance and vascular resistance, and neprilysin inhibitors have shown beneficial effects in patients with heart failure. However, the potential predictive value of neprilysin levels as a biomarker for cardiovascular risk remains unclear. The aim of this study was to assess the prognostic value of soluble neprilysin (sNEP) levels in patients with ischemic heart disease. Methods: Neprilysin levels were measured in 694 consecutive patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI). These patients were classified into two groups according to their serum levels of neprilysin and categorized into the lower neprilysin group (n = 348) and the higher neprilysin group (n = 346). The primary clinical endpoint was all-cause mortality, and the secondary endpoint was a composite of major adverse cardiac events (MACE). Results: The median sNEP level was 76.0 pg/ml. The median sNEP levels were higher in patients with left ventricular ejection fraction (LVEF) ≥40% (77.6 pg/ml, interquartile range 46.6–141.3) than in those with LVEF < 40% (70.0 pg/ml, interquartile range 47.1–100.6; P = 0.032). Among all patients, each clinical outcome and MACE did not differ significantly according to the groups divided into median, tertile, or quartile of sNEP levels during a median follow-up of 28.4 months. -

Stromal CD10 Expression in Breast Cancer Correlates with Tumor Invasion and Cancer Stem Cell Phenotype
Louhichi et al. BMC Cancer (2018) 18:49 DOI 10.1186/s12885-017-3951-8 RESEARCH ARTICLE Open Access Stromal CD10 expression in breast cancer correlates with tumor invasion and cancer stem cell phenotype Tahani Louhichi, Hanene Saad, Myriam Ben Dhiab, Sonia Ziadi and Mounir Trimeche* Abstract Background: Previous investigations have indicated that CD10 is associated with biological aggressivity in human cancers, but the use of this marker for diagnosis and prognosis is more complex. The aim of this study was to evaluate the expression of CD10 in breast cancer and its association with the clinicopathological features. In addition, we investigated whether a relationship exists between CD10 expression and cancer stem cells. Methods: CD10 expression was examined by the immunohistochemistry in a series of 133 invasive breast carcinoma cases. Results were correlated to several clinicopathological parameters. Cancer stem cell phenotype was assessed by the immunohistochemical analysis of CD44 and ALDH1. Results: Significant CD10 expression was found in the fusiform stromal cells in 19.5% of the cases and in the neoplastic cells in 7% of the cases. The stromal CD10 positivity was more frequently found in tumors with lymph node metastasis (p = 0.01) and a high histological grade (p = 0.01). However, CD10 expression by the neoplastic cells correlates with a high histological grade (p = 0.03) and the absence of estrogen (p = 0.002) as well as progesterone (p = 0.001) receptor expression. We also found that CD10 expression by the stromal cells, but not by the neoplastic cells, correlates significantly with the expression of cancer stem cell markers (CD44+/ALDH1+) (p = 0.002). -

Angiotensin Receptor Neprilysin Inhibition (ARNI) Following Acute Myocardial Infarction: Primary Results of the PARADISE-MI Trial Marc A
Angiotensin Receptor Neprilysin Inhibition (ARNI) Following Acute Myocardial Infarction: Primary Results of the PARADISE-MI Trial Marc A. Pfeffer, MD, PhD Distinguished Dzau Professor of Medicine Harvard Medical School Cardiovascular Division, Brigham and Women’s Hospital for the PARADISE-MI Committees, National Leaders and Investigators SAVE AIRE TRACE Radionuclide Clinical and/or Echocardiographic EF ≤ 40% radiographic signs EF ≤ 35% (1992) of HF (1993) (1995) 0.4 All-Cause Mortality 0.35 0.3 0.25 Placebo ACE-I 0.2 0.15 Placebo: 866/2971 (29.1%) Probability of of Probability Event 0.1 ACE-I: 702/2995 (23.4%) 0.05 OR: 0.74 (0.66–0.83) 0 Years 0 1 2 3 4 ACE-I 2995 2250 1617 892 223 Placebo 2971 2184 1521 853 138 Flather MD, et al. Lancet. 2000;355:1575–1581 Mortality in SAVE, TRACE, AIRE, and VALIANT Favors Active Drug Pfeffer,Pfeffer, McMurray, McMurray, Velazquez, Velazquez, et etal. al. N NEngl Engl J MedJ Med2003;3492003;349 2014 40 Enalapril 1117 32 (n=4212) 914 24 LCZ696 (n=4187) Meier Estimate of Meier Estimate 16 - HR = 0.80 (0.73-0.87) Cumulative (%) Rates Cumulative 8 Kaplan P = 0.0000002 Number needed to treat = 21 0 0 180 360 540 720 900 1080 1260 Patients at Risk Days After Randomization LCZ696 4187 3922 3663 3018 2257 1544 896 249 Enalapril 4212 3883 3579 2922 2123 1488 853 236 McMurray, N Engl J Med. 2014 AMI (0.5-7 days with LVEF ≤40% and/or pulmonary congestion) PLUS any risk enhancer Age ≥70 years Atrial fibrillation eGFR <60 LVEF < 30% Diabetes Killip class ≥III Prior MI STEMI without reperfusion Major Exclusions: Prior HF Clinical instability eGFR <30 Sacubitril/Valsartan Ramipril No run-in Target 97/103 mg BID Target 5 mg BID double-blind -controlled N=2830 active N=2831 Event driven: 711 primary endpoints Median follow-up: 23 months Primary Endpoint: CV death, HF hospitalization, outpatient development of HF Jering, Eur J ACC.21 Secondary Endpoint: CV death or first HF hospitalization Heart Fail. -

Epigenetic Suppression of Neprilysin Regulates Breast Cancer Invasion
OPEN Citation: Oncogenesis (2016) 5, e207; doi:10.1038/oncsis.2016.16 www.nature.com/oncsis ORIGINAL ARTICLE Epigenetic suppression of neprilysin regulates breast cancer invasion HM Stephen, RJ Khoury, PR Majmudar, T Blaylock1, K Hawkins1, MS Salama1, MD Scott1, B Cosminsky1, NK Utreja1, J Britt and RE Conway In women, invasive breast cancer is the second most common cancer and the second cause of cancer-related death. Therefore, identifying novel regulators of breast cancer invasion could lead to additional biomarkers and therapeutic targets. Neprilysin, a cell-surface enzyme that cleaves and inactivates a number of substrates including endothelin-1 (ET1), has been implicated in breast cancer, but whether neprilysin promotes or inhibits breast cancer cell progression and metastasis is unclear. Here, we asked whether neprilysin expression predicts and functionally regulates breast cancer cell invasion. RT–PCR and flow cytometry analysis of MDA-MB-231 and MCF-7 breast cancer cell lines revealed decreased neprilysin expression compared with normal epithelial cells. Expression was also suppressed in invasive ductal carcinoma (IDC) compared with normal tissue. In addition, in vitro invasion assays demonstrated that neprilysin overexpression decreased breast cancer cell invasion, whereas neprilysin suppression augmented invasion. Furthermore, inhibiting neprilysin in MCF-7 breast cancer cells increased ET1 levels significantly, whereas overexpressing neprilysin decreased extracellular-signal related kinase (ERK) activation, indicating that neprilysin negatively regulates ET1-induced activation of mitogen-activated protein kinase (MAPK) signaling. To determine whether neprilysin was epigenetically suppressed in breast cancer, we performed bisulfite conversion analysis of breast cancer cells and clinical tumor samples. We found that the neprilysin promoter was hypermethylated in breast cancer; chemical reversal of methylation in MDA-MB-231 cells reactivated neprilysin expression and inhibited cancer cell invasion. -

R&D Assay for Alzheimer's Disease
R&DR&D assayassay forfor Alzheimer’sAlzheimer’s diseasedisease Target screening⳼ Ⲽ㬔 antibody array, ᢜ⭉㬔 ⸽ἐⴐ Amyloid β-peptide Alzheimer’s disease⯸ ኸᷠ᧔ ᆹ⸽ inhibitor, antibody, ELISA kit Surwhrph#Surilohu#Dqwlerg|#Duud| 6OUSFBUFE 1."5SFBUFE )41 $3&# &3, &3, )41 $3&# &3, &3, 壤伡庰䋸TBNQMF ɅH 侴䋸嵄䍴䋸BOBMZUFT䋸䬱娴哜塵 1$ 1$ 1$ 1$ 5IFNPTUSFGFSFODFEBSSBZT 1$ 1$ QQ α 34, .4, 503 Q α 34, .4, 503 %SVHTDSFFOJOH0òUBSHFUFòFDUT0ATHWAY涭廐 6OUSFBUFE 堄币䋸4BNQMF侴䋸8FTUFSOPS&-*4"䍘䧽 1."5SFBUFE P 8FTUFSOCMPU廽喜儤应侴䋸0, Z 4VCTUSBUF -JHIU )31DPOKVHBUFE1BO "OUJQIPTQIPUZSPTJOF .FBO1JYFM%FOTJUZ Y $BQUVSF"OUJCPEZ 5BSHFU"OBMZUF "SSBZ.FNCSBOF $3&# &3, &3, )41 .4, Q α 34, 503 Human XL Cytokine Array kit (ARY022, 102 analytes) Adiponectin,Aggrecan,Angiogenin,Angiopoietin-1,Angiopoietin-2,BAFF,BDNF,Complement,Component C5/C5a,CD14,CD30,CD40L, Chitinase 3-like 1,Complement Factor D,C-Reactive Protein,Cripto-1,Cystatin C,Dkk-1,DPPIV,EGF,EMMPRIN,ENA-78,Endoglin, Fas L,FGF basic,FGF- 7,FGF-19,Flt-3 L,G-CSF,GDF-15,GM-CSF,GRO-α,Grow th Hormone,HGF,ICAM-1,IFN-γ,IGFBP-2,IGFBP-3, IL-1α,IL-1β, IL-1ra,IL-2,IL-3,IL-4,IL- 5,IL-6,IL-8, IL-10,IL-11,IL-12, IL-13,IL-15,IL-16,IL-17A,IL-18 BPa,IL-19,IL-22, IL-23,IL-24,IL-27, IL-31,IL-32α/β/γ,IL-33,IL-34,IP-10,I-TAC,Kallikrein 3,Leptin,LIF,Lipocalin-2,MCP-1,MCP-3,M-CSF,MIF,MIG,MIP-1α/MIP-1β,MIP-3α,MIP-3β,MMP-9, Myeloperoxidase,Osteopontin, p70, PDGF-AA, PDGF-AB/BB,Pentraxin-3, PF4, RAGE, RANTES,RBP4,Relaxin-2, Resistin,SDF-1α,Serpin E1, SHBG, ST2, TARC,TFF3,TfR,TGF- ,Thrombospondin-1,TNF-α, uPAR, VEGF, Vitamin D BP Human Protease (34 analytes) / -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -
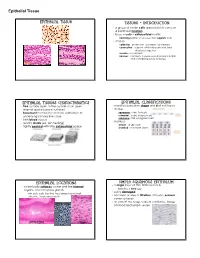
Epithelial Tissue
Epithelial Tissue Epithelial Tissue Tissues - Introduction · a group of similar cells specialized to carry on a particular function · tissue = cells + extracellular matrix nonliving portion of a tissue that supports cells · 4 types epithelial - protection, secretion, absorption connective - support soft body parts and bind structures together muscle - movement nervous - conducts impulses used to help control and coordinate body activities Epithelial Tissues Characteristics Epithelial Classifications · free surface open to the outside or an open · classified based on shape and # of cell layers internal space (apical surface) · shape · basement membrane anchors epithelium to squamous - thin, flat cells underlying connective tissue cuboidal - cube-shaped cells columnar - tall, elongated cells · lack blood vessels · number · readily divide (ex. skin healing) simple - single layer · tightly packed with little extracellular space stratified - 2 or more layers Epithelial Locations Simple Squamous Epithelium · a single layer of thin, flattened cells · cover body surfaces, cover and line internal organs, and compose glands looks like a fried egg · easily damaged skin cells, cells that line the stomach and small intestine, inside your mouth · common at sites of filtration, diffusion, osmosis; cover surfaces · air sacs of the lungs, walls of capillaries, linings cheek cells of blood and lymph vessels intestines skin Epithelial Tissue Simple Cuboidal Epithelium Simple Columnar Epithelium · single layer of cube-shaped cells · single layer of cells -

Common Single-Nucleotide Polymorphisms in the Estrogen Receptor B Promoter Are Associated with Colorectal Cancer Survival in Postmenopausal Women
Published OnlineFirst November 13, 2012; DOI: 10.1158/0008-5472.CAN-12-2484 Cancer Prevention and Epidemiology Research Common Single-Nucleotide Polymorphisms in the Estrogen Receptor b Promoter Are Associated with Colorectal Cancer Survival in Postmenopausal Women Michael N. Passarelli1,2, Amanda I. Phipps1, John D. Potter1,2,3, Karen W. Makar1, Anna E. Coghill1,2, Karen J. Wernli3,4, Emily White1,2, Andrew T. Chan5,6, Carolyn M. Hutter1,2, Ulrike Peters1,2, and Polly A. Newcomb1,2 Abstract Loss of estrogen receptor b (ERb) expression in the gut is associated with colorectal cancer (CRC) initiation and progression. Germline single-nucleotide polymorphisms (SNP) in genes for the sex-steroid hormone receptors are not strongly associated with CRC risk; however, these SNPs have not previously been evaluated in relation to survival after diagnosis. We enrolled 729 women, ages 50 to 74, diagnosed with invasive CRC between 1997 and 2002 in 13 counties covered by the Seattle-Puget Sound Surveillance Epidemiology and End Results cancer registry. Participants provided germline DNA. We selected 99 tag-SNPs for the androgen receptor (AR), ERa (ESR1), ERb (ESR2), and progesterone receptor (PGR) genes. Mortality outcomes were ascertained from the National Death Index. During a median of 6.6 years of follow-up, 244 deaths occurred (161 from CRC). We identified 20 SNPs (12 of ESR2 and 8 of PGR) for replication in 1,729 women diagnosed with incident invasive CRC (555 deaths; 405 from CRC) from three prospective cohort studies that participate in the Genetics and Epidemiology of Colorectal Cancer Consortium. Three correlated SNPs in the promoter of ESR2 (rs2987983, rs3020443, and rs2978381) were statistically significant predictors of CRC-specific and overall survival. -

Irf1) Signaling Regulates Apoptosis and Autophagy to Determine Endocrine Responsiveness and Cell Fate in Human Breast Cancer
INTERFERON REGULATORY FACTOR-1 (IRF1) SIGNALING REGULATES APOPTOSIS AND AUTOPHAGY TO DETERMINE ENDOCRINE RESPONSIVENESS AND CELL FATE IN HUMAN BREAST CANCER A Dissertation Submitted to the Faculty of the Graduate School of Arts and Sciences of Georgetown University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Physiology & Biophysics By Jessica L. Roberts, B.S. Washington, DC September 27, 2013 Copyright 2013 by Jessica L. Roberts All Rights Reserved ii INTERFERON REGULATORY FACTOR-1 (IRF1) SIGNALING REGULATES APOPTOSIS AND AUTOPHAGY TO DETERMINE ENDOCRINE RESPONSIVENESS AND CELL FATE IN HUMAN BREAST CANCER Jessica L. Roberts, B.S. Thesis Advisor: Robert Clarke, Ph.D. ABSTRACT Interferon regulatory factor-1 (IRF1) is a nuclear transcription factor and pivotal regulator of cell fate in cancer cells. While IRF1 is known to possess tumor suppressive activities, the role of IRF1 in mediating apoptosis and autophagy in breast cancer is largely unknown. Here, we show that IRF1 inhibits antiapoptotic B-cell lymphoma 2 (BCL2) protein expression, whose overexpression often contributes to antiestrogen resistance. We proposed that directly targeting the antiapoptotic BCL2 members with GX15-070 (GX; obatoclax), a BH3-mimetic currently in clinical development, would be an attractive strategy to overcome antiestrogen resistance in some breast cancers. Inhibition of BCL2 activity, through treatment with GX, was more effective in reducing the cell density of antiestrogen resistant breast cancer cells versus sensitive cells, and this increased sensitivity correlated with an accumulation of autophagic vacuoles. While GX treatment promoted autophagic vacuole and autolysosome formation, p62/SQSTM1, a marker for autophagic degradation, levels accumulated.