Catford Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Belowground Insect Herbivory Mediates Invasion Dynamics and Impact of an Exotic Plant
plants Article Release from Above- and Belowground Insect Herbivory Mediates Invasion Dynamics and Impact of an Exotic Plant Lotte Korell 1,2,3,4,* , Martin Schädler 3,4, Roland Brandl 5, Susanne Schreiter 6 and Harald Auge 3,4 1 Plant Ecology and Geobotany, Department of Ecology, University of Marburg, Karl-von-Frisch-Str. 8, 35032 Marburg, Germany 2 Institute of Biology, Martin Luther University Halle-Wittenberg, Am Kirchtor 1, 06108 Halle (Saale), Germany 3 Department of Community Ecology, Helmholtz-Centre for Environmental Research -UFZ, Theodor-Lieser-Str. 4, 06120 Halle, Germany; [email protected] (M.S.); [email protected] (H.A.) 4 German Centre for Integrative Biodiversity Research (iDiv), Halle-Jena-Leipzig, Deutscher Platz 5e, 04103 Leipzig, Germany 5 Animal Ecology, Department of Ecology, University of Marburg, Karl-von-Frisch-Str. 8, 35032 Marburg, Germany; [email protected] 6 Department of Soil System Science, Helmholtz-Centre for Environmental Research - UFZ, Theodor-Lieser-Str. 4, 06120 Halle, Germany; [email protected] * Correspondence: [email protected] Received: 28 October 2019; Accepted: 21 November 2019; Published: 26 November 2019 Abstract: The enemy-release hypothesis is one of the most popular but also most discussed hypotheses to explain invasion success. However, there is a lack of explicit, experimental tests of predictions of the enemy-release hypothesis (ERH), particularly regarding the effects of above- and belowground herbivory. Long-term studies investigating the relative effect of herbivores on invasive vs. native plant species within a community are still lacking. Here, we report on a long-term field experiment in an old-field community, invaded by Solidago canadensis s. -

Shell Crushing Resistance of Alien and Native Thiarid Gastropods to Predatory Crabs in South Africa
Aquatic Invasions (2016) Volume 11, Issue 3: 303–311 DOI: http://dx.doi.org/10.3391/ai.2016.11.3.08 Open Access © 2016 The Author(s). Journal compilation © 2016 REABIC Research Article Shell crushing resistance of alien and native thiarid gastropods to predatory crabs in South Africa 1, 2 1 1 1 Nelson A.F. Miranda *, G. John Measey , Nasreen Peer , Jacqueline L. Raw , Renzo Perissinotto 3 and Christopher C. Appleton 1DST/NRF Research Chair in Shallow Water Ecosystems, Nelson Mandela Metropolitan University, Port Elizabeth 6031, South Africa 2Centre for Invasion Biology, Department of Botany & Zoology, Stellenbosch University, Stellenbosch, South Africa 3School of Life Sciences, Westville Campus, University of KwaZulu-Natal, Durban, South Africa *Corresponding author E-mail: [email protected] Received: 22 September 2015 / Accepted: 10 March 2016 / Published online: 11 April 2016 Handling editor: Kenneth Hayes Abstract The successful invasion of freshwater and coastal lakes of South Africa by the recently introduced thiarid snail Tarebia granifera may be due in part to release from predatory pressure. This study aimed to determine the comparative vulnerability of T. granifera and the widespread native aquatic thiarid Melanoides tuberculata to predation. These species also account for many thiarid invasions in the Americas, Europe and parts of Africa. We quantified the shell crushing resistance of these snails, as well as the maximal shell crushing capability of native freshwater crab predators, Potamonautes sidneyi and P. perlatus. Using an Instron isometric transducer, we showed that Tarebia granifera shells were significantly stronger than Melanoides shells, and exceeded the crushing strength we documented for both potential predatory crabs. -

Lionfish Parasite Release
Biol Invasions (2017) 19:563–575 DOI 10.1007/s10530-016-1342-8 ORIGINAL PAPER Parasite-mediated enemy release and low biotic resistance may facilitate invasion of Atlantic coral reefs by Pacific red lionfish (Pterois volitans) Lillian J. Tuttle . Paul C. Sikkel . Katherine Cure . Mark A. Hixon Received: 6 November 2015 / Accepted: 28 November 2016 / Published online: 8 December 2016 Ó Springer International Publishing Switzerland 2016 Abstract Successful invasions are largely explained Bahamas and the Cayman Islands) than at two regions by some combination of enemy release, where the in their native Pacific range (the Northern Marianas invader escapes its natural enemies from its native Islands and the Philippines). This pattern was largely range, and low biotic resistance, where native species driven by relatively high infection rates of lionfish by in the introduced range fail to control the invader. We didymozoan fluke worms and parasitic copepods examined the extent to which parasites may mediate (which may be host-specific to Pterois lionfishes) in both release and resistance in the introduction of the Marianas and the Philippines, respectively. When Pacific red lionfish (Pterois volitans) to Atlantic coral compared with sympatric, native fishes in the Atlantic, reefs. We found that fewer lionfish were parasitized at invasive lionfish were at least 18 times less likely to two regions in their introduced Atlantic range (The host a parasite in The Bahamas and at least 40 times less likely to host a parasite in the Cayman Islands. We found no indication that lionfish introduced Pacific Electronic supplementary material The online version of parasites into the Atlantic. -

Testing the Enemy Release Hypothesis in a Native Insect Species with an Expanding Range
Testing the enemy release hypothesis in a native insect species with an expanding range Julia J. Mlynarek Biology Department, University of Biology, Fredericton, New Brunswick, Canada ABSTRACT The enemy release hypothesis (ERH) predicts that the spread of (invasive) species will be facilitated by release from their enemies as they occupy new areas. How- ever, the ERH is rarely tested on native (non-invasive, long established) species with expanding or shifting ranges. I tested the ERH for a native damselfly (Enallagma clausum) whose range has recently expanded in western Canada, with respect to its water mite and gregarine parasites. Parasitism levels (prevalence and intensity) were also compared between E. clausum and a closely related species, Enallagma boreale, which has long been established in the study region and whose range is not shifting. A total of 1,150 damselflies were collected at three ‘old’ sites for E. clausum in Saskatchewan, and three ‘new’ sites in Alberta. A little more than a quarter of the damselflies collected were parasitized with, on average, 18 water mite individuals, and 20% were parasitized by, on average, 10 gregarine individuals. I assessed whether the diVerences between levels of infection (prevalence and intensity) were due to site type or host species. The ERH was not supported: Enallagma clausum has higher or the same levels of parasitism in new sites than old sites. However, E. boreale seems to be benefitting from the recent range expansion of a native, closely related species through ecological release from its parasites because the parasites may be choosing to infest the novel, potentially na¨ıve, host instead of the well-established host. -

Negative Soil Feedbacks Accumulate Over Time for Nonnative Plant Species
Ecology Letters, (2010) 13: 803–809 doi: 10.1111/j.1461-0248.2010.01474.x LETTER Negative soil feedbacks accumulate over time for non-native plant species Abstract Jeffrey M. Diez,1* Ian Dickie,2 The enemy release hypothesis is a common explanation for species invasions, suggesting Grant Edwards,1 Philip E. Hulme,1 that introduced species benefit from leaving behind natural enemies in the native range. Jon J. Sullivan1 and Richard P. However, any such advantage may attenuate over time. In this study, we test a prediction 1 Duncan of this more dynamic enemy release hypothesis: that non-native plant species that 1 Bio-Protection Research became established longer ago exhibit stronger negative feedbacks with the soil. Centre, PO Box 84, Lincoln Consistent with declining enemy release over time, we found increasingly negative soil University, Lincoln 7647, New feedbacks for species established longer ago in New Zealand. Negative soil feedbacks Zealand 2 were also stronger for more widespread species, but weaker for more locally abundant Landcare Research, PO Box 40, Lincoln 7640, New Zealand species, suggesting that species accumulate negative interactions as they spread and can *Correspondence and present be locally regulated by these interactions. We also present data to support the common address: E-mail: assumption that relatives have similar impacts on and responses to soil communities. [email protected]; School of Together, these data highlight the dynamic nature of novel interactions arising from Natural Resources and species introductions. Environment, University of Michigan, 440 Church St, Ann Keywords Arbor, MI 48109 1041, USA Enemy release hypothesis, invasive species, plant–soil feedbacks. -
A Review and Meta-Analysis of the Enemy Release Hypothesis in Plant–Herbivorous Insect Systems
A peer-reviewed version of this preprint was published in PeerJ on 21 December 2016. View the peer-reviewed version (peerj.com/articles/2778), which is the preferred citable publication unless you specifically need to cite this preprint. Meijer K, Schilthuizen M, Beukeboom L, Smit C. 2016. A review and meta- analysis of the enemy release hypothesis in plant–herbivorous insect systems. PeerJ 4:e2778 https://doi.org/10.7717/peerj.2778 A review and meta-analysis of the enemy release hypothesis in plant–herbivorous insect systems Kim Meijer 1, 2 , Menno Schilthuizen 1, 3 , Leo Beukeboom 1 , Christian Smit Corresp. 1 1 Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, The Netherlands 2 Altenburg & Wymenga Ecological Consultants, Veenwouden, the Netherlands 3 Endless Forms group, Naturalis Biodiversity Center, Leiden, Netherlands Corresponding Author: Christian Smit Email address: [email protected] A suggested mechanism for the success of introduced non-native species is the enemy release hypothesis (ERH). Many studies have tested the predictions of the ERH using the community approach (native and non-native species studied in the same habitat) or the biogeographical approach (species studied in their native and non-native range), but results are highly variable, possibly due to large variety of study systems incorporated. We therefore focused on one specific system: plants and their herbivorous insects. We performed a systematic review and compiled a large number (68) of datasets from studies comparing herbivorous insects on native and non-native plants using the community or biogeographical approach. We performed a meta-analysis to test the predictions from the ERH for insect diversity (number of species), insect load (number of individuals) and level of herbivory for both the community and biogeographical approach. -
Indirect Effects of Host-Specific Biological Control Agents
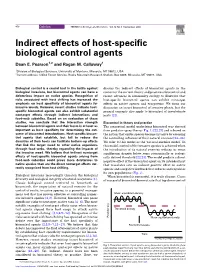
456 Opinion TRENDS in Ecology and Evolution Vol.18 No.9 September 2003 Indirect effects of host-specific biological control agents Dean E. Pearson1,2 and Ragan M. Callaway1 1Division of Biological Sciences, University of Montana, Missoula, MT 59812, USA 2Current address: USDA Forest Service, Rocky Mountain Research Station, Box 8089, Missoula, MT 59807, USA Biological control is a crucial tool in the battle against discuss the indirect effects of biocontrol agents in the biological invasions, but biocontrol agents can have a context of the current theory and practice of biocontrol and deleterious impact on native species. Recognition of recent advances in community ecology to illustrate that risks associated with host shifting has increased the host-specific biocontrol agents can exhibit nontarget emphasis on host specificity of biocontrol agents for effects on native species and ecosystems. We focus our invasive weeds. However, recent studies indicate host- discussion on insect biocontrol of invasive plants, but the specific biocontrol agents can also exhibit substantial general concepts also apply to biocontrol of invertebrate nontarget effects through indirect interactions and pests [21]. food-web subsidies. Based on an evaluation of these studies, we conclude that the interaction strength Biocontrol in theory and practice between biocontrol agents and their hosts is at least as The conceptual model underlying biocontrol was derived important as host specificity for determining the out- from predator–prey theory (Fig. 1) [22,23] and is based on come of biocontrol introductions. Host-specific biocon- the notion that exotic species become invasive by escaping trol agents that establish, but fail to reduce the the controlling influence of their natural enemies [24–26]. -

The Endophyte-Enemy Release Hypothesis: Implications for Classical Biological Control and Plant Invasions
The endophyte-enemy release hypothesis: implications for classical biological control and plant invasions H.C. Evans Summary Fungal endophytes are asymptomless colonizers of higher plants for all, or a part, of their life cycles. They range from latent pathogens to symbionts. There is increasing evidence that some form mutually beneficial, highly specialized or co-evolved associations with their hosts and that they provide the plant with an additional armoury to combat abiotic and biotic stresses, including pests and diseases. Thus, there may be a trade-off between reduced growth (in the short term), as nutrients are sequestered by the fungal mutualist, but increased overall long-term fitness as natural-enemy pressure is decreased. This tripartite balance may be lost when plants arrive in exotic ecosystems with incomplete guilds of both co-evolved endophytes and natural enemies. The enemy release hypothesis (ERH) explains why alien plants can become invasive. It is now hypothesized that another, more cryptic but still significant factor could also be involved: the presence or absence of mutualistic endophytes. Those neophytes ar- riving without co-evolved natural enemies but with mutualistic co-evolved endophytes would have a double advantage over local competitors. Such endophytic-enriched, alien-invasive weeds and those that form mutualistic associations with indigenous endophytes could help to explain the inconsisten- cies of some classical biological control introductions. Similarly, those alien plants that arrive and remain endophyte-free and without co-evolved natural enemies would have a distinct competitive advantage because they would have more resources to allocate to growth and reproduction, given, of course, that there are no significant pressures from indigenous natural enemies or that sufficient auto-defences are retained to overcome them. -

Escape from Parasites
Chapter 11 Escape from Parasites Mark E. Torchin and Kevin D. Lafferty 11.1 Introduction In betting circles, the odds-on favorite of a sporting match can depend on the health of the star players; a pulled hamstring or bad flu can determine the winner. Introduced species are often in contest with native species and their impacts are directly proportional to their demographic performance in the novel environment. Demographic performance can encompass population level parameters, such as densities, abundances, and biomass as well as individual level parameters, such as growth rate, survivorship and fecundity. One indication of an invader’s demo- graphic performance is size because individuals that grow fast or live long can become large. On average, marine invaders attain larger sizes compared to popula- tions in their native range (Grosholz and Ruiz 2003). This increased performance, if it translates into increased standing biomass, should positively correlate with an invader’s impact (Crivelli 1983). Exploring reasons for invasion success will not only accelerate our understanding of species interactions, but will strengthen our ability to manage invasions. Debate as to what factors facilitate invasion success has led to quantitative evalu- ation of a long-standing explanation for successful introduced species: the enemy release hypothesis (ERH). One prediction of the ERH is that introduced popula- tions lack natural enemies compared to populations within their original range (Williams 1954; Elton 1958). Another prediction of the ERH is that introduced species should benefit from enemy-mediated competition because they are less likely to be affected by natural enemies than their native competitors (Elton 1958; Keane and Crawley 2002). -
Community Assembly Theory As a Framework for Biological Invasions
Opinion Community Assembly Theory as a Framework for Biological Invasions 1,2, 1 3 4,5 Dean E. Pearson, * Yvette K. Ortega, Özkan Eren, and José L. Hierro Biological invasions present a global problem underlain by an ecological para- Highlights dox that thwarts explanation: how do some exotic species, evolutionarily naïve We build on recent studies to demon- strate how community assembly the- to their new environments, outperform locally adapted natives? We propose ory can provide a framework for that community assembly theory provides a framework for addressing this advancing invasion ecology. question. Local community assembly rules can be defined by evaluating In this framework, the success of exo- how native species’ traits interact with community filters to affect species tic species in recipient communities is abundance. Evaluation of exotic species against this benchmark indicates that determined by how their traits interact with local ecological filters. exotics that follow assembly rules behave like natives, while those exhibiting novel interactions with community filters can greatly underperform or outper- This approach illustrates that most form natives. Additionally, advantages gained by exotics over natives following exotics follow community rules to become naturalized species that disturbance can be explained by accounting for extrinsic assembly processes behave like the natives; that is, traits that bias exotic traits toward ruderal strategies. predict their abundance according to local rules as they do for natives. How- ever, some exotics can underperform or outperform relative to the natives The Need for an Overarching Framework to Guide Invasion Ecology when they exhibit novel interactions Human-assisted translocations of biological organisms have enhanced societies around the with local filters due to their unique globe by increasing the availability and diversity of foods, medicines, and construction materials ecological–evolutionary histories. -
Endophytes of Invasive Weeds: Pertinence to Classical Biological Control in India
Journal of Biological Control, 29(3): 115-120, 2015 Review Article Endophytes of invasive weeds: pertinence to classical biological control in India PRAKYA SREERAMA KUMAR ICAR–National Bureau of Agricultural Insect Resources, P.O. Box 2491, H.A. Farm Post, Hebbal, Bengaluru 560 024, India. E-mail: [email protected] ABSTRACT: Suppression of the water weed Salvinia molesta in India and control of the woody-perennial Cryptostegia grandiflora in Australia are two of the finest successes achieved through classical biological control (CBC). There is no guarantee, nevertheless, that CBC should always be successful in every situation. Though the enemy release hypothesis explains why invasive alien species thrive in exotic locations, there has never been a convincing explanation on the poor performance of some introduced natural enemies, in spite of the rigorous screening for their potential. Interestingly, Evans’ endophyte–enemy release hypothesis provides enough clarification by implicating the absence of protective coevolved endophytes in weed populations in exotic locations. This review gives an introduction to endophytes and analyses the pertinence of the newly proposed hypothesis to CBC of weeds in India with examples. KEY WORDS: Classical biological control, endophytes, natural enemies, weed management (Article chronicle: Received: 09-09-2015; Revised: 14-09-2015; Accepted: 16-09-2015) INTRODUCTION does not always guarantee complete control of the weed species. For example, out of the nine species of insects and Globally, 224 weeds have so far been targeted with 551 two rust fungi introduced into Australia to control parthe- biocontrol agents (Winston et al., 2014). Some of the finest nium weed (Parthenium hysterophorus), seven species of successes in weed biocontrol have been achieved through insects and both rusts have successfully established as bio- classical biological control (CBC). -
Cadotte 1.Indd
Chapter eleven Use of biological invasions and their control to study the dynamics of interacting populations F. Courchamp and S. Caut INTRODUCTION One of the difficulties of conservation biology is the general lack of experimental approaches. Because it is often unethical, or simply because this new discipline deals with small and/or fragile populations, experiments on those populations are not always feasible. As a result, the knowledge on population dynamics, when not dealing with laboratory populations of caged invertebrates, has often come from theoretical studies, with notable exceptions such as those based on of some populations isolated on particular islands (e.g., (Clutton-Brock and Coulson, 2002; Grenfell et al., 1998). However, one aspect that is often lacking from theoretical studies, as well as from natural isolated populations, is the interspecific dimension: in the above cases, it is rather exceptional to take into account more than two interacting populations. Yet, as we hope to show in this chapter, direct and indirect “complex” interspecific relationships may be the major ecological forces in some communities. They can thus be crucial for applied ecology as well M.W. Cadotte, S.M. McMahon and T. Fukami (eds), Conceptual ecology and invasions biology, 253–279. © 2005 Springer. Printed in Great Britain. 254 Dynamics of interacting populations as represent heuristic tools for students, and intellectual candies for functional ecologists. However, there is an enormous set of ecological events that can be viewed as natural, large-scale experiments: biological invasions. Several aspects make bio- logical invasions an interesting tool for the study of interspecific interactions: they are of various types, involve many different organisms, and happen in contrasted ecosystems.