Lionfish Parasite Release
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zoology Marine Ornamental Fish Biodiversity of West Bengal ABSTRACT
Research Paper Volume : 4 | Issue : 8 | Aug 2015 • ISSN No 2277 - 8179 Zoology Marine Ornamental Fish Biodiversity of KEYWORDS : Marine fish, ornamental, West Bengal diversity, West Bengal. Principal Scientist and Scientist-in-Charge, ICAR-Central Institute of Fisheries Education, Dr. B. K. Mahapatra Salt Lake City, Kolkata-700091, India Director and Vice-Chancellor, ICAR-Central Institute of Fisheries Education, Versova, Dr. W. S. Lakra Mumbai- 400 061, India ABSTRACT The State of West Bengal, India endowed with 158 km coast line for marine water resources with inshore, up-shore areas and continental shelf of Bay of Bengal form an important fishery resource and also possesses a rich wealth of indigenous marine ornamental fishes.The present study recorded a total of 113 marine ornamental fish species, belonging to 75 genera under 45 families and 10 orders.Order Perciformes is represented by a maximum of 26 families having 79 species under 49 genera followed by Tetraodontiformes (5 family; 9 genus and 10 species), Scorpaeniformes (2 family; 3 genus and 6 species), Anguilliformes (2 family; 3 genus and 4 species), Syngnathiformes (2 family; 3 genus and 3 species), Pleuronectiformes (2 family; 2 genus and 4 species), Siluriformes (2 family; 2 genus and 3 species), Beloniformes (2 family; 2 genus and 2 species), Lophiformes (1 family; 1 genus and 1 species), Beryciformes(1 family; 1 genus and 1 species). Introduction Table 1: List of Marine ornamental fishes of West Bengal Ornamental fishery, which started centuries back as a hobby, ORDER 1: PERCIFORMES has now started taking the shape of a multi-billion dollar in- dustry. -

And Belowground Insect Herbivory Mediates Invasion Dynamics and Impact of an Exotic Plant
plants Article Release from Above- and Belowground Insect Herbivory Mediates Invasion Dynamics and Impact of an Exotic Plant Lotte Korell 1,2,3,4,* , Martin Schädler 3,4, Roland Brandl 5, Susanne Schreiter 6 and Harald Auge 3,4 1 Plant Ecology and Geobotany, Department of Ecology, University of Marburg, Karl-von-Frisch-Str. 8, 35032 Marburg, Germany 2 Institute of Biology, Martin Luther University Halle-Wittenberg, Am Kirchtor 1, 06108 Halle (Saale), Germany 3 Department of Community Ecology, Helmholtz-Centre for Environmental Research -UFZ, Theodor-Lieser-Str. 4, 06120 Halle, Germany; [email protected] (M.S.); [email protected] (H.A.) 4 German Centre for Integrative Biodiversity Research (iDiv), Halle-Jena-Leipzig, Deutscher Platz 5e, 04103 Leipzig, Germany 5 Animal Ecology, Department of Ecology, University of Marburg, Karl-von-Frisch-Str. 8, 35032 Marburg, Germany; [email protected] 6 Department of Soil System Science, Helmholtz-Centre for Environmental Research - UFZ, Theodor-Lieser-Str. 4, 06120 Halle, Germany; [email protected] * Correspondence: [email protected] Received: 28 October 2019; Accepted: 21 November 2019; Published: 26 November 2019 Abstract: The enemy-release hypothesis is one of the most popular but also most discussed hypotheses to explain invasion success. However, there is a lack of explicit, experimental tests of predictions of the enemy-release hypothesis (ERH), particularly regarding the effects of above- and belowground herbivory. Long-term studies investigating the relative effect of herbivores on invasive vs. native plant species within a community are still lacking. Here, we report on a long-term field experiment in an old-field community, invaded by Solidago canadensis s. -

Journal Threatened
Journal ofThreatened JoTT TBuilding evidenceaxa for conservation globally 10.11609/jott.2020.12.1.15091-15218 www.threatenedtaxa.org 26 January 2020 (Online & Print) Vol. 12 | No. 1 | 15091–15218 ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) PLATINUM OPEN ACCESS ISSN 0974-7907 (Online); ISSN 0974-7893 (Print) Publisher Host Wildlife Information Liaison Development Society Zoo Outreach Organization www.wild.zooreach.org www.zooreach.org No. 12, Thiruvannamalai Nagar, Saravanampatti - Kalapatti Road, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Ph: +91 9385339863 | www.threatenedtaxa.org Email: [email protected] EDITORS English Editors Mrs. Mira Bhojwani, Pune, India Founder & Chief Editor Dr. Fred Pluthero, Toronto, Canada Dr. Sanjay Molur Mr. P. Ilangovan, Chennai, India Wildlife Information Liaison Development (WILD) Society & Zoo Outreach Organization (ZOO), 12 Thiruvannamalai Nagar, Saravanampatti, Coimbatore, Tamil Nadu 641035, Web Design India Mrs. Latha G. Ravikumar, ZOO/WILD, Coimbatore, India Deputy Chief Editor Typesetting Dr. Neelesh Dahanukar Indian Institute of Science Education and Research (IISER), Pune, Maharashtra, India Mr. Arul Jagadish, ZOO, Coimbatore, India Mrs. Radhika, ZOO, Coimbatore, India Managing Editor Mrs. Geetha, ZOO, Coimbatore India Mr. B. Ravichandran, WILD/ZOO, Coimbatore, India Mr. Ravindran, ZOO, Coimbatore India Associate Editors Fundraising/Communications Dr. B.A. Daniel, ZOO/WILD, Coimbatore, Tamil Nadu 641035, India Mrs. Payal B. Molur, Coimbatore, India Dr. Mandar Paingankar, Department of Zoology, Government Science College Gadchiroli, Chamorshi Road, Gadchiroli, Maharashtra 442605, India Dr. Ulrike Streicher, Wildlife Veterinarian, Eugene, Oregon, USA Editors/Reviewers Ms. Priyanka Iyer, ZOO/WILD, Coimbatore, Tamil Nadu 641035, India Subject Editors 2016–2018 Fungi Editorial Board Ms. Sally Walker Dr. B. Shivaraju, Bengaluru, Karnataka, India Founder/Secretary, ZOO, Coimbatore, India Prof. -

Shell Crushing Resistance of Alien and Native Thiarid Gastropods to Predatory Crabs in South Africa
Aquatic Invasions (2016) Volume 11, Issue 3: 303–311 DOI: http://dx.doi.org/10.3391/ai.2016.11.3.08 Open Access © 2016 The Author(s). Journal compilation © 2016 REABIC Research Article Shell crushing resistance of alien and native thiarid gastropods to predatory crabs in South Africa 1, 2 1 1 1 Nelson A.F. Miranda *, G. John Measey , Nasreen Peer , Jacqueline L. Raw , Renzo Perissinotto 3 and Christopher C. Appleton 1DST/NRF Research Chair in Shallow Water Ecosystems, Nelson Mandela Metropolitan University, Port Elizabeth 6031, South Africa 2Centre for Invasion Biology, Department of Botany & Zoology, Stellenbosch University, Stellenbosch, South Africa 3School of Life Sciences, Westville Campus, University of KwaZulu-Natal, Durban, South Africa *Corresponding author E-mail: [email protected] Received: 22 September 2015 / Accepted: 10 March 2016 / Published online: 11 April 2016 Handling editor: Kenneth Hayes Abstract The successful invasion of freshwater and coastal lakes of South Africa by the recently introduced thiarid snail Tarebia granifera may be due in part to release from predatory pressure. This study aimed to determine the comparative vulnerability of T. granifera and the widespread native aquatic thiarid Melanoides tuberculata to predation. These species also account for many thiarid invasions in the Americas, Europe and parts of Africa. We quantified the shell crushing resistance of these snails, as well as the maximal shell crushing capability of native freshwater crab predators, Potamonautes sidneyi and P. perlatus. Using an Instron isometric transducer, we showed that Tarebia granifera shells were significantly stronger than Melanoides shells, and exceeded the crushing strength we documented for both potential predatory crabs. -

National Invasive Lionfish Prevention and Management Plan
5/5/2014 National Invasive Lionfish Prevention and Management Plan PREPARED BY: INVASIVE LIONFISH CONTROL AD-HOC COMMITTEE OF THE AQUATIC NUISANCE SPECIES TASK FORCE ABBREVIATIONS LIST ANS – Aquatic Nuisance Species ANSTF – Aquatic Nuisance Species Task Force EDRR – Early Detection and Rapid Response FWC – Florida Fish and Wildlife Conservation Commission FKNMS – Florida Keys National Marine Sanctuary GSARP – Gulf and South Atlantic Regional Panel GSMFC – Gulf States Marine Fisheries Commission NAS – Non-Native Aquatic Species NGOs – Non-Governmental Organization PLAN – National Invasive Lionfish Prevention and Management Plan NISA – National Invasive Species Act of 1996 NMFS – National Marine Fisheries Service NOAA – National Oceanic and Atmospheric Administration NPS – National Park Service REEF – Reef Environmental Education Foundation USFWS – U.S. Fish and Wildlife Service USGS – U.S. Geological Survey TABLE OF CONTENTS EXECUTIVE SUMMARY ...................................................................................................................................... I COMMITTEE MEMBER LIST ........................................................................................................................... II LIST OF FIGURES ............................................................................................................................................... III LIST OF TABLES ................................................................................................................................................ -

Marine and Estuarine Fish Fauna of Tamil Nadu, India
Proceedings of the International Academy of Ecology and Environmental Sciences, 2018, 8(4): 231-271 Article Marine and estuarine fish fauna of Tamil Nadu, India 1,2 3 1 1 H.S. Mogalekar , J. Canciyal , D.S. Patadia , C. Sudhan 1Fisheries College and Research Institute, Thoothukudi - 628 008, Tamil Nadu, India 2College of Fisheries, Dholi, Muzaffarpur - 843 121, Bihar, India 3Central Inland Fisheries Research Institute, Barrackpore, Kolkata - 700 120, West Bengal, India E-mail: [email protected] Received 20 June 2018; Accepted 25 July 2018; Published 1 December 2018 Abstract Varied marine and estuarine ecosystems of Tamil Nadu endowed with diverse fish fauna. A total of 1656 fish species under two classes, 40 orders, 191 families and 683 geranra reported from marine and estuarine waters of Tamil Nadu. In the checklist, 1075 fish species were primary marine water and remaining 581 species were diadromus. In total, 128 species were reported under class Elasmobranchii (11 orders, 36 families and 70 genera) and 1528 species under class Actinopterygii (29 orders, 155 families and 613 genera). The top five order with diverse species composition were Perciformes (932 species; 56.29% of the total fauna), Tetraodontiformes (99 species), Pleuronectiforms (77 species), Clupeiformes (72 species) and Scorpaeniformes (69 species). At the family level, the Gobiidae has the greatest number of species (86 species), followed by the Carangidae (65 species), Labridae (64 species) and Serranidae (63 species). Fishery status assessment revealed existence of 1029 species worth for capture fishery, 425 species worth for aquarium fishery, 84 species worth for culture fishery, 242 species worth for sport fishery and 60 species worth for bait fishery. -

Risk Assessment for the Lionfish Pterois Miles (Bennett, 1828)
Risk Assessment for the lionfish Pterois miles (Bennett, 1828) Prepared in the framework of the EU LIFE project “Preventing a LIONfish invasion in the MEDiterranean through early response and targeted REmoval (RELIONMED-LIFE)” - LIFE16 NAT/CY/000832 EU NON-NATIVE ORGANISM RISK ASSESSMENT SCHEME Name of organism: Pterois miles (Bennett, 1828) Lead authors: Periklis Kleitou1, Ioannis Savva2, Demetris Kletou2 Contributing authors: Charalampos Antoniou2, Niki Chartosia3, Yiannis Christodoulides4,5, Maria Christou2, Louis Hadjioannou4, Margarita Hadjistylli5, Jason M Hall-Spencer1, Carlos Jimenez4, Sian Rees1 1 School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK 2 Marine and Environmental Research (MER) Lab., Limassol, 4533, Cyprus 3 Department of Biological Sciences University of Cyprus P.O. Box 20537, 1678 Nicosia, Cyprus. 4 Enalia Physis Environmental Research Centre, Acropoleos 2, Aglantzia 2101, Nicosia, Cyprus 5 Department of Environment, Ministry of Agriculture, Rural Development and Environment, Nicosia, Cyprus Risk Assessment Area: The geographical coverage of the risk assessment is the territory of the European Union (excluding outermost regions) and the United Kingdom Peer review 1: Stelios Katsanevakis, Dr, Department of Marine Sciences, University of the Aegean, Mytilene, Lesvos Island, Greece Peer review 2: Ernesto Azzurro, Dr, Institute for Environmental Protection and Research (ISPRA) Livorno, Italy Positive opinion by the Scientific Forum: 17/11/2020 1 | P a g e EU CHAPEAU QUESTION RESPONSE COMMENT -

Testing the Enemy Release Hypothesis in a Native Insect Species with an Expanding Range
Testing the enemy release hypothesis in a native insect species with an expanding range Julia J. Mlynarek Biology Department, University of Biology, Fredericton, New Brunswick, Canada ABSTRACT The enemy release hypothesis (ERH) predicts that the spread of (invasive) species will be facilitated by release from their enemies as they occupy new areas. How- ever, the ERH is rarely tested on native (non-invasive, long established) species with expanding or shifting ranges. I tested the ERH for a native damselfly (Enallagma clausum) whose range has recently expanded in western Canada, with respect to its water mite and gregarine parasites. Parasitism levels (prevalence and intensity) were also compared between E. clausum and a closely related species, Enallagma boreale, which has long been established in the study region and whose range is not shifting. A total of 1,150 damselflies were collected at three ‘old’ sites for E. clausum in Saskatchewan, and three ‘new’ sites in Alberta. A little more than a quarter of the damselflies collected were parasitized with, on average, 18 water mite individuals, and 20% were parasitized by, on average, 10 gregarine individuals. I assessed whether the diVerences between levels of infection (prevalence and intensity) were due to site type or host species. The ERH was not supported: Enallagma clausum has higher or the same levels of parasitism in new sites than old sites. However, E. boreale seems to be benefitting from the recent range expansion of a native, closely related species through ecological release from its parasites because the parasites may be choosing to infest the novel, potentially na¨ıve, host instead of the well-established host. -

Negative Soil Feedbacks Accumulate Over Time for Nonnative Plant Species
Ecology Letters, (2010) 13: 803–809 doi: 10.1111/j.1461-0248.2010.01474.x LETTER Negative soil feedbacks accumulate over time for non-native plant species Abstract Jeffrey M. Diez,1* Ian Dickie,2 The enemy release hypothesis is a common explanation for species invasions, suggesting Grant Edwards,1 Philip E. Hulme,1 that introduced species benefit from leaving behind natural enemies in the native range. Jon J. Sullivan1 and Richard P. However, any such advantage may attenuate over time. In this study, we test a prediction 1 Duncan of this more dynamic enemy release hypothesis: that non-native plant species that 1 Bio-Protection Research became established longer ago exhibit stronger negative feedbacks with the soil. Centre, PO Box 84, Lincoln Consistent with declining enemy release over time, we found increasingly negative soil University, Lincoln 7647, New feedbacks for species established longer ago in New Zealand. Negative soil feedbacks Zealand 2 were also stronger for more widespread species, but weaker for more locally abundant Landcare Research, PO Box 40, Lincoln 7640, New Zealand species, suggesting that species accumulate negative interactions as they spread and can *Correspondence and present be locally regulated by these interactions. We also present data to support the common address: E-mail: assumption that relatives have similar impacts on and responses to soil communities. [email protected]; School of Together, these data highlight the dynamic nature of novel interactions arising from Natural Resources and species introductions. Environment, University of Michigan, 440 Church St, Ann Keywords Arbor, MI 48109 1041, USA Enemy release hypothesis, invasive species, plant–soil feedbacks. -
A Review and Meta-Analysis of the Enemy Release Hypothesis in Plant–Herbivorous Insect Systems
A peer-reviewed version of this preprint was published in PeerJ on 21 December 2016. View the peer-reviewed version (peerj.com/articles/2778), which is the preferred citable publication unless you specifically need to cite this preprint. Meijer K, Schilthuizen M, Beukeboom L, Smit C. 2016. A review and meta- analysis of the enemy release hypothesis in plant–herbivorous insect systems. PeerJ 4:e2778 https://doi.org/10.7717/peerj.2778 A review and meta-analysis of the enemy release hypothesis in plant–herbivorous insect systems Kim Meijer 1, 2 , Menno Schilthuizen 1, 3 , Leo Beukeboom 1 , Christian Smit Corresp. 1 1 Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, The Netherlands 2 Altenburg & Wymenga Ecological Consultants, Veenwouden, the Netherlands 3 Endless Forms group, Naturalis Biodiversity Center, Leiden, Netherlands Corresponding Author: Christian Smit Email address: [email protected] A suggested mechanism for the success of introduced non-native species is the enemy release hypothesis (ERH). Many studies have tested the predictions of the ERH using the community approach (native and non-native species studied in the same habitat) or the biogeographical approach (species studied in their native and non-native range), but results are highly variable, possibly due to large variety of study systems incorporated. We therefore focused on one specific system: plants and their herbivorous insects. We performed a systematic review and compiled a large number (68) of datasets from studies comparing herbivorous insects on native and non-native plants using the community or biogeographical approach. We performed a meta-analysis to test the predictions from the ERH for insect diversity (number of species), insect load (number of individuals) and level of herbivory for both the community and biogeographical approach. -
Indirect Effects of Host-Specific Biological Control Agents
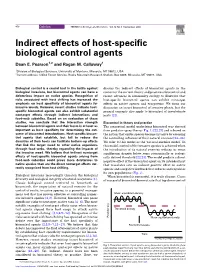
456 Opinion TRENDS in Ecology and Evolution Vol.18 No.9 September 2003 Indirect effects of host-specific biological control agents Dean E. Pearson1,2 and Ragan M. Callaway1 1Division of Biological Sciences, University of Montana, Missoula, MT 59812, USA 2Current address: USDA Forest Service, Rocky Mountain Research Station, Box 8089, Missoula, MT 59807, USA Biological control is a crucial tool in the battle against discuss the indirect effects of biocontrol agents in the biological invasions, but biocontrol agents can have a context of the current theory and practice of biocontrol and deleterious impact on native species. Recognition of recent advances in community ecology to illustrate that risks associated with host shifting has increased the host-specific biocontrol agents can exhibit nontarget emphasis on host specificity of biocontrol agents for effects on native species and ecosystems. We focus our invasive weeds. However, recent studies indicate host- discussion on insect biocontrol of invasive plants, but the specific biocontrol agents can also exhibit substantial general concepts also apply to biocontrol of invertebrate nontarget effects through indirect interactions and pests [21]. food-web subsidies. Based on an evaluation of these studies, we conclude that the interaction strength Biocontrol in theory and practice between biocontrol agents and their hosts is at least as The conceptual model underlying biocontrol was derived important as host specificity for determining the out- from predator–prey theory (Fig. 1) [22,23] and is based on come of biocontrol introductions. Host-specific biocon- the notion that exotic species become invasive by escaping trol agents that establish, but fail to reduce the the controlling influence of their natural enemies [24–26]. -

The Endophyte-Enemy Release Hypothesis: Implications for Classical Biological Control and Plant Invasions
The endophyte-enemy release hypothesis: implications for classical biological control and plant invasions H.C. Evans Summary Fungal endophytes are asymptomless colonizers of higher plants for all, or a part, of their life cycles. They range from latent pathogens to symbionts. There is increasing evidence that some form mutually beneficial, highly specialized or co-evolved associations with their hosts and that they provide the plant with an additional armoury to combat abiotic and biotic stresses, including pests and diseases. Thus, there may be a trade-off between reduced growth (in the short term), as nutrients are sequestered by the fungal mutualist, but increased overall long-term fitness as natural-enemy pressure is decreased. This tripartite balance may be lost when plants arrive in exotic ecosystems with incomplete guilds of both co-evolved endophytes and natural enemies. The enemy release hypothesis (ERH) explains why alien plants can become invasive. It is now hypothesized that another, more cryptic but still significant factor could also be involved: the presence or absence of mutualistic endophytes. Those neophytes ar- riving without co-evolved natural enemies but with mutualistic co-evolved endophytes would have a double advantage over local competitors. Such endophytic-enriched, alien-invasive weeds and those that form mutualistic associations with indigenous endophytes could help to explain the inconsisten- cies of some classical biological control introductions. Similarly, those alien plants that arrive and remain endophyte-free and without co-evolved natural enemies would have a distinct competitive advantage because they would have more resources to allocate to growth and reproduction, given, of course, that there are no significant pressures from indigenous natural enemies or that sufficient auto-defences are retained to overcome them.