Elucidation of the Extended Pedigree of 'Honeycrisp' Apple and Genetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

RGC8-S7O03 H. Muranty.Pdf
Analysis of genetic control of fruit size in apple using both multiple, pedigree-related and single full- sib families Hélène MURANTY, François LAURENS, Marco C.A.M. BINK , Eric van de WEG Hélène MURANTY RGC8 21-23 June 2016 Introduction • Fruit size = appearance + yield component • QTL for marker-assisted selection – large population precise location – large diversity consistency across genetic backgrounds Pedigree-based analysis • Bink et al 2002, 2008, 2014 • Rosyara et al 2013 • Fresnedo-Ramírez et al 2015, 2016 • Roach et al 2016 • Allard et al 2016 2 Hélène MURANTY RGC8 21-23 June2 2016 Previous studies reference cross pop fruit trait linkage group size 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Liebhard et al Discovery × Fiesta 251 weight (2003) X X X X X X X X Kenis et al (2008) Telamon × Braeburn 199 / weight, X X X X X X X 165 diameter, height Royal Gala × 572 Devoghalaere et al Braeburn weight X X X X X X (2012) Starkrimson × 123 Granny Smith Chang et al (2014) Jonathan × Golden 144 / weight, X X X X Delicious 140 diameter, length Fuji × Delearly 86 Fuji × Cripps Pink 73 size, weight, Costa (2015) Golden Delicious × 185 X X X X X X X Scarlet diameter, height Golden Delicious × 75 Braeburn 3 Hélène MURANTY RGC8 21-23 June3 2016 Material Z185 BVIII_34.16 GoldenDel X-4355 X-6820 PRI612-1 F_X-4355 Generos 12_E AntonovkaOB Chantecler Idared Florina X-6681 Delicious PRI672-3 X-6683 26 related F_B8_34.16 X-3177 Baujade PRI14-126 ReiDuMans I_M Clochard PRI14-152 X-6799 TN_R10A8 families X-3259 X-6398 HiDRAS Jonathan Winesap Crandall -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

Diseases of Tree Fruit Apple: Diagnosis and Management
1 Diseases of Tree Fruit Apple: Diagnosis and Management Sara M. Villani June 22, 2017 Department of Entomology and Plant Pathology, NCSU [email protected] 2 Apple Disease Challenges in the S.E. • Several apple diseases to contend with Apple Disease Challenges in the S.E. 3 • Paucity of disease resistant cultivars – Breeding efforts focus on consumer preference – Usually single-disease resistance ‘Enterprise’ ‘Prima’ ‘Goldrush’ http://www.eatlikenoone.com/prima-apples.htm http://www.eatlikenoone.com/enterpris-apples.htm http://kuffelcreek.wordpress.com/ ‘William’s Pride’ ‘Liberty’ ‘Pristine’ http://www.eatlikenoone.com/pristine-apples.htm http://www.plant.photos.net/index.php?title=File:Apple_williams_pride.jpg http://www.plant.photos.net/index.php?title=File:Apple_libertye.jpg Apple Disease Challenges in the S.E. 4 • Warm, humid climate – Favorable for pathogen infection and disease development – Inadequate chilling hours: longer period of susceptibility to blossom infection Susceptible Host Biology and Conducive availability of Environment pathogen Apple Disease Challenges in the S.E. 5 • Maintaining practices of fungicide resistance management and maximum annual applications – Commercial apple growers in Hendersonville NC: Up to 24 fungicide applications in 2017! Multi-site Single-site Biologicals Protectants Fungicides Mancozeb Group 3: S.I.’s Bacillus spp. Captan Group 11: “Strobys” A. pullulans Copper Group 7: SDHIs Sulfur Group 1: “T-Methyl” ziram U12: Dodine Phosphorous Acid Confusing Fungicide Jargon 6 Fungicides are classified in a number of ways: 1. Chemical Group – e.g. triazoles, benzimidazoles 2. Biochemical Mode of Action (my preference, common in academia) – e.g. Demethylation inhibitor (DMI); Quinone-outside inhibitor (QoI) 3. Physical Mode of Action – e.g. -
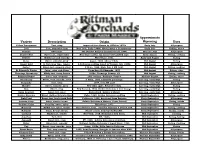
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Canadian Viagra Generic
Edible Landscape Nursery at Central Rocky Mountain Permaculture Institute (CRMPI) www.crmpi.org/projects/nursery www.coloradopear.com Additional Fruit/ Container Currently quantities Common Name Rootstock Price Function Size available available summer 2018 Apple Akane + Greening double graft #7 Domestic $65 1 apple apple Apple Alexander apple #10-#15 Domestic $55 1 apple Apple Arkcharm apple #10-#15 Domestic $55 1 apple Apple E. German Rome apple #10-#15 Antonovka $55 3 apple Apple Green Rabbit apple #10-#15 Domestic $55 3 apple Apple Greening apple #10-#15 Domestic $55 1 apple Apple Lodi apple #10-#15 Domestic $55 2 apple Apple Mac Fence apple #10-#15 Domestic $55 1 apple Apple Nanette apple #6-#7 Antonovka $55 3 apple Apple New Mac apple / Novamac #10-#15 Domestic $55 2 apple Apple Northern Spy apple #10-#15 M111 semi- $75 1 dwarf apple Apple Red Baron apple #10-#15 Domestic $55 1 apple Apple Red Gravenstein apple #10-#15 M111 semi- $75 5 dwarf apple Apple Sweet Sixteen apple #10-#15 Antonovka $55 2 apple Apple Yellow transparent apple #10-#15 Antonovka $55 1 apple Apple Wolf River + Gold Rush apple #10-#15 Antonovka $75 1 double graft apple Antique apple Colorado Orange + Gold Rush #10-#15 Antonovka $65 1 double graft apple apple Antique apple Colorado Orange heritage apple #10-#15 Antonovka $55 1 apple Antique apple Gold Rush apple #10-#15 Antonovka $55 2 apple Antique apple Royal Limber + Pomme Gris #10-#15 Domestic $65 1 double graft apple apple Antique apple Winter Banana heritage apple #10-#15 Domestic $55 3 apple Page 1 of 4 -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

Vyhláška Č. 331/2017 Sb
zakonyprolidi_cs_2017_331_v20180124 https://www.zakonyprolidi.cz/print/cs/2017-331/zneni-20180124.htm Vyhláška č. 331/2017 Sb. Vyhláška o stanovení dalších odrůdovocných druhů s úředně uznaným popisem, které se považují za zapsané do Státní odrůdové knihy https://www.zakonyprolidi.cz/cs/2017-331 Částka 113/2017 Platnost od 11.10.2017 Účinnost od 01.11.2017 Aktuální znění 24.01.2018 331 VYHLÁŠKA ze dne 2. října 2017 o stanovení dalších odrůd ovocných druhů s úředně uznaným popisem, které se považují za zapsané do Státní odrůdové knihy Ministerstvo zemědělství stanoví podle § 35c odst. 5 zákona č. 219/2003 Sb., o uvádění do oběhu osiva a sadby pěstovaných rostlin a o změně některých zákonů (zákon o oběhu osiva a sadby), ve znění zákona č. 295/2017 Sb.: § 1 Další odrůdy ovocných druhů s úředně uznaným popisem, které se považují za zapsané do Státní odrůdové knihy, jsou uvedeny v příloze k této vyhlášce. § 2 Účinnost Tato vyhláška nabývá účinnosti dnem 1. listopadu 2017. Ministr: Ing. Jurečka v. r. Příloha k vyhlášce č. 331/2017 Sb. Seznam dalších odrůd ovocných druhů s úředně uznaným popisem, které se považují za zapsané do Státní odrůdové knihy Druh Odrůda Líska (Corylus avellana L.) Lombardská červená Římský Kdouloň (Cydonia oblonga Milí.) Asenica Bereczkého Hruškovitá Izobilnaja Kocurova Leskovačka Muškatnaja Selena Jahodník (Fragaria L.) Evita Frikonsa Kama 1 z 11 07.03.2018, 13:22 zakonyprolidi_cs_2017_331_v20180124 https://www.zakonyprolidi.cz/print/cs/2017-331/zneni-20180124.htm Lesana Maranell Mount Everest Olivie Polka Roxana Vanda -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL. -

アーティスト 商品名 品番 ジャンル名 定価 URL 100% (Korea) RE
アーティスト 商品名 品番 ジャンル名 定価 URL 100% (Korea) RE:tro: 6th Mini Album (HIP Ver.)(KOR) 1072528598 K-POP 2,290 https://tower.jp/item/4875651 100% (Korea) RE:tro: 6th Mini Album (NEW Ver.)(KOR) 1072528759 K-POP 2,290 https://tower.jp/item/4875653 100% (Korea) 28℃ <通常盤C> OKCK05028 K-POP 1,296 https://tower.jp/item/4825257 100% (Korea) 28℃ <通常盤B> OKCK05027 K-POP 1,296 https://tower.jp/item/4825256 100% (Korea) 28℃ <ユニット別ジャケット盤B> OKCK05030 K-POP 648 https://tower.jp/item/4825260 100% (Korea) 28℃ <ユニット別ジャケット盤A> OKCK05029 K-POP 648 https://tower.jp/item/4825259 100% (Korea) How to cry (Type-A) <通常盤> TS1P5002 K-POP 1,204 https://tower.jp/item/4415939 100% (Korea) How to cry (Type-B) <通常盤> TS1P5003 K-POP 1,204 https://tower.jp/item/4415954 100% (Korea) How to cry (ミヌ盤) <初回限定盤>(LTD) TS1P5005 K-POP 602 https://tower.jp/item/4415958 100% (Korea) How to cry (ロクヒョン盤) <初回限定盤>(LTD) TS1P5006 K-POP 602 https://tower.jp/item/4415970 100% (Korea) How to cry (ジョンファン盤) <初回限定盤>(LTD) TS1P5007 K-POP 602 https://tower.jp/item/4415972 100% (Korea) How to cry (チャンヨン盤) <初回限定盤>(LTD) TS1P5008 K-POP 602 https://tower.jp/item/4415974 100% (Korea) How to cry (ヒョクジン盤) <初回限定盤>(LTD) TS1P5009 K-POP 602 https://tower.jp/item/4415976 100% (Korea) Song for you (A) OKCK5011 K-POP 1,204 https://tower.jp/item/4655024 100% (Korea) Song for you (B) OKCK5012 K-POP 1,204 https://tower.jp/item/4655026 100% (Korea) Song for you (C) OKCK5013 K-POP 1,204 https://tower.jp/item/4655027 100% (Korea) Song for you メンバー別ジャケット盤 (ロクヒョン)(LTD) OKCK5015 K-POP 602 https://tower.jp/item/4655029 100% (Korea) -

Washington Apple Pi Journal, May 1988
Wa1hington Journal of WasGhington Appl e Pi, Ltd Volume. 10 may 1988 number 5 Hiahliaht.1 ' . ~ The Great Apple Lawsuit- (pgs4&so) • Timeout AppleWorks Enhancements - I IGs •Personal Newsletter & Publish It! ~ MacNovice: Word Processing- The Next Generati on ~ Views & Reviews: MidiPaint and Performer ~ Macintosh Virus: Technical Notes In This Issue. Officers & Staff, Editorial ................................ ...................... 3 Personal Newsletter and Publish It! .................... Ray Settle 40* President's Corner ........................................... Tom Warrick 4 • GameSIG News ....................................... Charles Don Hall 41 Event Queue, General Information, Classifieds ..................... 6 Plan for Use of Tutorial Tapes & Disks ................................ 41 AV-SIG News .............................................. Nancy Sefcrian 6 The Design Page for W.A.P .................................. Jay Rohr 42 Commercial Classifieds, Job Mart .......................................... 7 MacNovice Column: Word Processing .... Ralph J. Begleiter 47 * WAP Calendar, SIG News ..................................................... 8 Developer's View: The Great Apple Lawsuit.. ..... Bill Hole 50• WAP Hotline............................................................. ............. 9 Macintosh Bits & Bytes ............................... Lynn R. Trusal 52 Q & A ............................... Robert C. Platt & Bruce F. Field 10 Macinations 3 .................................................. Robb Wolov 56 Using