Contents 1 Shell Model of the Nucleus I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arxiv:Nucl-Th/0402046V1 13 Feb 2004
The Shell Model as Unified View of Nuclear Structure E. Caurier,1, ∗ G. Mart´ınez-Pinedo,2,3, † F. Nowacki,1, ‡ A. Poves,4, § and A. P. Zuker1, ¶ 1Institut de Recherches Subatomiques, IN2P3-CNRS, Universit´eLouis Pasteur, F-67037 Strasbourg, France 2Institut d’Estudis Espacials de Catalunya, Edifici Nexus, Gran Capit`a2, E-08034 Barcelona, Spain 3Instituci´oCatalana de Recerca i Estudis Avan¸cats, Llu´ıs Companys 23, E-08010 Barcelona, Spain 4Departamento de F´ısica Te´orica, Universidad Aut´onoma, Cantoblanco, 28049, Madrid, Spain (Dated: October 23, 2018) The last decade has witnessed both quantitative and qualitative progresses in Shell Model stud- ies, which have resulted in remarkable gains in our understanding of the structure of the nucleus. Indeed, it is now possible to diagonalize matrices in determinantal spaces of dimensionality up to 109 using the Lanczos tridiagonal construction, whose formal and numerical aspects we will analyze. Besides, many new approximation methods have been developed in order to overcome the dimensionality limitations. Furthermore, new effective nucleon-nucleon interactions have been constructed that contain both two and three-body contributions. The former are derived from realistic potentials (i.e., consistent with two nucleon data). The latter incorporate the pure monopole terms necessary to correct the bad saturation and shell-formation properties of the real- istic two-body forces. This combination appears to solve a number of hitherto puzzling problems. In the present review we will concentrate on those results which illustrate the global features of the approach: the universality of the effective interaction and the capacity of the Shell Model to describe simultaneously all the manifestations of the nuclear dynamics either of single particle or collective nature. -

A Suggestion Complementing the Magic Numbers Interpretation of the Nuclear Fission Phenomena
World Journal of Nuclear Science and Technology, 2018, 8, 11-22 http://www.scirp.org/journal/wjnst ISSN Online: 2161-6809 ISSN Print: 2161-6795 A Suggestion Complementing the Magic Numbers Interpretation of the Nuclear Fission Phenomena Faustino Menegus F. Menegus V. Europa, Bussero, Italy How to cite this paper: Menegus, F. Abstract (2018) A Suggestion Complementing the Magic Numbers Interpretation of the Nu- Ideas, solely related on the nuclear shell model, fail to give an interpretation of clear Fission Phenomena. World Journal of the experimental central role of 54Xe in the asymmetric fission of actinides. Nuclear Science and Technology, 8, 11-22. The same is true for the β-delayed fission of 180Tl to 80Kr and 100Ru. The repre- https://doi.org/10.4236/wjnst.2018.81002 sentation of the natural isotopes, in the Z-Neutron Excess plane, suggests the Received: November 21, 2017 importance of the of the Neutron Excess evolution mode in the fragments of Accepted: January 23, 2018 the asymmetric actinide fission and in the fragments of the β-delayed fission Published: January 26, 2018 of 180Tl. The evolution mode of the Neutron Excess, hinged at Kr and Xe, is Copyright © 2018 by author and directed by the 50 and 82 neutron magic numbers. The present isotope repre- Scientific Research Publishing Inc. sentation offers a frame for the interpretation of the post fission evaporation This work is licensed under the Creative of neutrons, higher for the AL compared to the AH fragments, a tenet in nuc- Commons Attribution International License (CC BY 4.0). lear fission. -

Nuclear Models: Shell Model
LectureLecture 33 NuclearNuclear models:models: ShellShell modelmodel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 III.III. ShellShell modelmodel 3 ShellShell modelmodel Magic numbers: Nuclides with certain proton and/or neutron numbers are found to be exceptionally stable. These so-called magic numbers are 2, 8, 20, 28, 50, 82, 126 — The doubly magic nuclei: — Nuclei with magic proton or neutron number have an unusually large number of stable or long lived nuclides . — A nucleus with a magic neutron (proton) number requires a lot of energy to separate a neutron (proton) from it. — A nucleus with one more neutron (proton) than a magic number is very easy to separate. — The first exitation level is very high : a lot of energy is needed to excite such nuclei — The doubly magic nuclei have a spherical form Nucleons are arranged into complete shells within the atomic nucleus 4 ExcitationExcitation energyenergy forfor magicm nuclei 5 NuclearNuclear potentialpotential The energy spectrum is defined by the nuclear potential solution of Schrödinger equation for a realistic potential The nuclear force is very short-ranged => the form of the potential follows the density distribution of the nucleons within the nucleus: for very light nuclei (A < 7), the nucleon distribution has Gaussian form (corresponding to a harmonic oscillator potential ) for heavier nuclei it can be parameterised by a Fermi distribution. -

Keynote Address: One Hundred Years of Nuclear Physics – Progress and Prospects
PRAMANA c Indian Academy of Sciences Vol. 82, No. 4 — journal of April 2014 physics pp. 619–624 Keynote address: One hundred years of nuclear physics – Progress and prospects S KAILAS1,2 1Bhabha Atomic Research Centre, Mumbai 400 085, India 2UM–DAE Centre for Excellence in Basic Sciences, Mumbai 400 098, India E-mail: [email protected] DOI: 10.1007/s12043-014-0710-0; ePublication: 5 April 2014 Abstract. Nuclear physics research is growing on several fronts, along energy and intensity fron- tiers, with exotic projectiles and targets. The nuclear physics facilities under construction and those being planned for the future make the prospects for research in this field very bright. Keywords. Nuclear structure and reactions; nuclear properties; superheavy nuclei. PACS Nos 21.10.–k; 25.70.Jj; 25.70.–z 1. Introduction Nuclear physics research is nearly one hundred years old. Currently, this field of research is progressing [1] broadly in three directions (figure 1): Investigation of nuclei and nuclear matter at high energies and densities; observation of behaviour of nuclei under extreme conditions of temperature, angular momentum and deformation; and production and study of nuclei away from the line of stability. Nuclear physics research began with the investiga- tion of about 300 nuclei. Today, this number has grown many folds, nearly by a factor of ten. In the area of high-energy nuclear physics, some recent phenomena observed have provided interesting connections to other disciplines in physics, e.g. in the heavy-ion collisions at relativistic energies, it has been observed [2] that the hot dense matter formed in the collision behaved like an ideal fluid with the ratio of shear viscosity to entropy being close to 1/4π. -

14. Structure of Nuclei Particle and Nuclear Physics
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14. Structure of Nuclei 2 Magic Numbers Magic Numbers = 2; 8; 20; 28; 50; 82; 126... Nuclei with a magic number of Z and/or N are particularly stable, e.g. Binding energy per nucleon is large for magic numbers Doubly magic nuclei are especially stable. Dr. Tina Potter 14. Structure of Nuclei 3 Magic Numbers Other notable behaviour includes Greater abundance of isotopes and isotones for magic numbers e.g. Z = 20 has6 stable isotopes (average=2) Z = 50 has 10 stable isotopes (average=4) Odd A nuclei have small quadrupole moments when magic First excited states for magic nuclei higher than neighbours Large energy release in α, β decay when the daughter nucleus is magic Spontaneous neutron emitters have N = magic + 1 Nuclear radius shows only small change with Z, N at magic numbers. etc... etc... Dr. Tina Potter 14. Structure of Nuclei 4 Magic Numbers Analogy with atomic behaviour as electron shells fill. Atomic case - reminder Electrons move independently in central potential V (r) ∼ 1=r (Coulomb field of nucleus). Shells filled progressively according to Pauli exclusion principle. Chemical properties of an atom defined by valence (unpaired) electrons. Energy levels can be obtained (to first order) by solving Schr¨odinger equation for central potential. 1 E = n = principle quantum number n n2 Shell closure gives noble gas atoms. Are magic nuclei analogous to the noble gas atoms? Dr. -

Three Related Topics on the Periodic Tables of Elements
Three related topics on the periodic tables of elements Yoshiteru Maeno*, Kouichi Hagino, and Takehiko Ishiguro Department of physics, Kyoto University, Kyoto 606-8502, Japan * [email protected] (The Foundations of Chemistry: received 30 May 2020; accepted 31 July 2020) Abstaract: A large variety of periodic tables of the chemical elements have been proposed. It was Mendeleev who proposed a periodic table based on the extensive periodic law and predicted a number of unknown elements at that time. The periodic table currently used worldwide is of a long form pioneered by Werner in 1905. As the first topic, we describe the work of Pfeiffer (1920), who refined Werner’s work and rearranged the rare-earth elements in a separate table below the main table for convenience. Today’s widely used periodic table essentially inherits Pfeiffer’s arrangements. Although long-form tables more precisely represent electron orbitals around a nucleus, they lose some of the features of Mendeleev’s short-form table to express similarities of chemical properties of elements when forming compounds. As the second topic, we compare various three-dimensional helical periodic tables that resolve some of the shortcomings of the long-form periodic tables in this respect. In particular, we explain how the 3D periodic table “Elementouch” (Maeno 2001), which combines the s- and p-blocks into one tube, can recover features of Mendeleev’s periodic law. Finally we introduce a topic on the recently proposed nuclear periodic table based on the proton magic numbers (Hagino and Maeno 2020). Here, the nuclear shell structure leads to a new arrangement of the elements with the proton magic-number nuclei treated like noble-gas atoms. -

Low-Energy Nuclear Physics Part 2: Low-Energy Nuclear Physics
BNL-113453-2017-JA White paper on nuclear astrophysics and low-energy nuclear physics Part 2: Low-energy nuclear physics Mark A. Riley, Charlotte Elster, Joe Carlson, Michael P. Carpenter, Richard Casten, Paul Fallon, Alexandra Gade, Carl Gross, Gaute Hagen, Anna C. Hayes, Douglas W. Higinbotham, Calvin R. Howell, Charles J. Horowitz, Kate L. Jones, Filip G. Kondev, Suzanne Lapi, Augusto Macchiavelli, Elizabeth A. McCutchen, Joe Natowitz, Witold Nazarewicz, Thomas Papenbrock, Sanjay Reddy, Martin J. Savage, Guy Savard, Bradley M. Sherrill, Lee G. Sobotka, Mark A. Stoyer, M. Betty Tsang, Kai Vetter, Ingo Wiedenhoever, Alan H. Wuosmaa, Sherry Yennello Submitted to Progress in Particle and Nuclear Physics January 13, 2017 National Nuclear Data Center Brookhaven National Laboratory U.S. Department of Energy USDOE Office of Science (SC), Nuclear Physics (NP) (SC-26) Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No.DE-SC0012704 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

Upper Limit in Mendeleev's Periodic Table Element No.155
AMERICAN RESEARCH PRESS Upper Limit in Mendeleev’s Periodic Table Element No.155 by Albert Khazan Third Edition — 2012 American Research Press Albert Khazan Upper Limit in Mendeleev’s Periodic Table — Element No. 155 Third Edition with some recent amendments contained in new chapters Edited and prefaced by Dmitri Rabounski Editor-in-Chief of Progress in Physics and The Abraham Zelmanov Journal Rehoboth, New Mexico, USA — 2012 — This book can be ordered in a paper bound reprint from: Books on Demand, ProQuest Information and Learning (University of Microfilm International) 300 N. Zeeb Road, P. O. Box 1346, Ann Arbor, MI 48106-1346, USA Tel.: 1-800-521-0600 (Customer Service) http://wwwlib.umi.com/bod/ This book can be ordered on-line from: Publishing Online, Co. (Seattle, Washington State) http://PublishingOnline.com Copyright c Albert Khazan, 2009, 2010, 2012 All rights reserved. Electronic copying, print copying and distribution of this book for non-commercial, academic or individual use can be made by any user without permission or charge. Any part of this book being cited or used howsoever in other publications must acknowledge this publication. No part of this book may be re- produced in any form whatsoever (including storage in any media) for commercial use without the prior permission of the copyright holder. Requests for permission to reproduce any part of this book for commercial use must be addressed to the Author. The Author retains his rights to use this book as a whole or any part of it in any other publications and in any way he sees fit. -

Nuclear Physics
Massachusetts Institute of Technology 22.02 INTRODUCTION to APPLIED NUCLEAR PHYSICS Spring 2012 Prof. Paola Cappellaro Nuclear Science and Engineering Department [This page intentionally blank.] 2 Contents 1 Introduction to Nuclear Physics 5 1.1 Basic Concepts ..................................................... 5 1.1.1 Terminology .................................................. 5 1.1.2 Units, dimensions and physical constants .................................. 6 1.1.3 Nuclear Radius ................................................ 6 1.2 Binding energy and Semi-empirical mass formula .................................. 6 1.2.1 Binding energy ................................................. 6 1.2.2 Semi-empirical mass formula ......................................... 7 1.2.3 Line of Stability in the Chart of nuclides ................................... 9 1.3 Radioactive decay ................................................... 11 1.3.1 Alpha decay ................................................... 11 1.3.2 Beta decay ................................................... 13 1.3.3 Gamma decay ................................................. 15 1.3.4 Spontaneous fission ............................................... 15 1.3.5 Branching Ratios ................................................ 15 2 Introduction to Quantum Mechanics 17 2.1 Laws of Quantum Mechanics ............................................. 17 2.2 States, observables and eigenvalues ......................................... 18 2.2.1 Properties of eigenfunctions ......................................... -
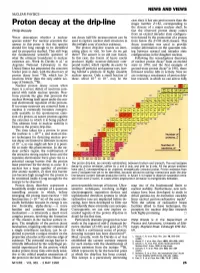
Proton Decay at the Drip-Line Magic Number Z = 82, Corresponding to the Closure of a Major Nuclear Shell
NEWS AND VIEWS NUCLEAR PHYSICS-------------------------------- case since it has one proton more than the Proton decay at the drip-line magic number Z = 82, corresponding to the closure of a major nuclear shell. In Philip Woods fact the observed proton decay comes from an excited intruder state configura WHAT determines whether a nuclear ton decay half-life measurements can be tion formed by the promotion of a proton species exists? For nuclear scientists the used to explore nuclear shell structures in from below the Z=82 shell closure. This answer to this poser is that the species this twilight zone of nuclear existence. decay transition was used to provide should live long enough to be identified The proton drip-line sounds an inter unique information on the quantum mix and its properties studied. This still begs esting place to visit. So how do we get ing between normal and intruder state the fundamental scientific question of there? The answer is an old one: fusion. configurations in the daughter nucleus. what the ultimate boundaries to nuclear In this case, the fusion of heavy nuclei Following the serendipitous discovery existence are. Work by Davids et al. 1 at produces highly neutron-deficient com of nuclear proton decay4 from an excited Argonne National Laboratory in the pound nuclei, which rapidly de-excite by state in 1970, and the first example of United States has pinpointed the remotest boiling off particles and gamma-rays, leav ground-state proton decay5 in 1981, there border post to date, with the discovery of ing behind a plethora of highly unstable followed relative lulls in activity. -

Nuclear Magic Numbers: New Features Far from Stability
Nuclear magic numbers: new features far from stability O. Sorlin1, M.-G. Porquet2 1Grand Acc´el´erateur National d’Ions Lours (GANIL), CEA/DSM - CNRS/IN2P3, B.P. 55027, F-14076 Caen Cedex 5, France 2Centre de Spectrom´etrie Nucl´eaire et de Spectrom´etrie de Masse (CSNSM), CNRS/IN2P3 - Universit´eParis-Sud, Bˆat 104-108, F-91405 Orsay, France May 19, 2008 Abstract The main purpose of the present manuscript is to review the structural evolution along the isotonic and isotopic chains around the ”traditional” magic numbers 8, 20, 28, 50, 82 and 126. The exotic regions of the chart of nuclides have been explored during the three last decades. Then the postulate of permanent magic numbers was definitely abandoned and the reason for these structural mutations has been in turn searched for. General trends in the evolution of shell closures are discussed using complementary experimental information, such as the binding energies of the orbits bounding the shell gaps, the trends of the first collective states of the even-even semi-magic nuclei, and the behavior of certain single-nucleon states. Each section is devoted to a particular magic number. It describes the underlying physics of the shell evolution which is not yet fully understood and indicates future experimental and theoretical challenges. The nuclear mean field embodies various facets of the Nucleon- Nucleon interaction, among which the spin-orbit and tensor terms play decisive roles in the arXiv:0805.2561v1 [nucl-ex] 16 May 2008 shell evolutions. The present review intends to provide experimental constraints to be used for the refinement of theoretical models aiming at a good description of the existing atomic nuclei and at more accurate predictions of hitherto unreachable systems. -

Father of the Shell Model
CENTENARY Father of the shell model Heidelberg University held a symposium on fundamental physics and the shell model this summer to celebrate the 100th anniversary of the birth of Hans Jensen, the German who created the nuclear shell model with Maria Goeppert-Mayer of Argonne. Hans Jensen (1907–1973) is the only theorist among the three winners from Heidelberg University of the Nobel Prize for Physics. He shared the award with Maria Goeppert-Mayer in 1963 for the development of the nuclear shell model, which they published independently in 1949. The model offered the first coherent expla- nation for the variety of properties and structures of atomic nuclei. In particular, the “magic numbers” of protons and neutrons, which had been determined experimentally from the stability properties and observed abundances of chemical elements, found a natural explanation in terms of the spin-orbit coupling of the nucleons. These numbers play a decisive role in the synthesis of the ele- ments in stars, as well as in the artificial synthesis of the heaviest elements at the borderline of the periodic table of elements. Hans Jensen was born in Hamburg on 25 June 1907. He studied physics, mathematics, chemistry and philosophy in Hamburg and Freiburg, obtaining his PhD in 1932. After a short period in the Hans Jensen in his study at 16 Philosophenweg, Heidelberg German army’s weather service, he became professor of theo- in 1963. (Courtesy Bettmann/UPI/Corbis.) retical physics in Hannover in 1940. Jensen then accepted a new chair for theoretical physics in Heidelberg in 1949 on the initiative Mayer 1949).