Oxygen Toxicity and Radiation Injury to the Pulmonary System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Job Hazard Analysis
Identifying and Evaluating Hazards in Research Laboratories Guidelines developed by the Hazards Identification and Evaluation Task Force of the American Chemical Society’s Committee on Chemical Safety Copyright 2013 American Chemical Society Table of Contents FOREWORD ................................................................................................................................................... 3 ACKNOWLEDGEMENTS ................................................................................................................................. 5 Task Force Members ..................................................................................................................................... 6 1. SCOPE AND APPLICATION ..................................................................................................................... 7 2. DEFINITIONS .......................................................................................................................................... 7 3. HAZARDS IDENTIFICATION AND EVALUATION ................................................................................... 10 4. ESTABLISHING ROLES AND RESPONSIBILITIES .................................................................................... 14 5. CHOOSING AND USING A TECHNIQUE FROM THIS GUIDE ................................................................. 17 6. CHANGE CONTROL .............................................................................................................................. 19 7. ASSESSING -

Infections of the Respiratory Tract
F70954-07.qxd 12/10/02 7:36 AM Page 71 Infections of the respiratory 7 tract the nasal hairs and by inertial impaction with mucus- 7.1 Pathogenesis 71 covered surfaces in the posterior nasopharynx (Fig. 11). 7.2 Diagnosis 72 The epiglottis, its closure reflex and the cough reflex all reduce the risk of microorganisms reaching the lower 7.3 Management 72 respiratory tract. Particles small enough to reach the tra- 7.4 Diseases and syndromes 73 chea and bronchi stick to the respiratory mucus lining their walls and are propelled towards the oropharynx 7.5 Organisms 79 by the action of cilia (the ‘mucociliary escalator’). Self-assessment: questions 80 Antimicrobial factors present in respiratory secretions further disable inhaled microorganisms. They include Self-assessment: answers 83 lysozyme, lactoferrin and secretory IgA. Particles in the size range 5–10 µm may penetrate further into the lungs and even reach the alveolar air Overview spaces. Here, alveolar macrophages are available to phagocytose potential pathogens, and if these are overwhelmed neutrophils can be recruited via the This chapter deals with infections of structures that constitute inflammatory response. The defences of the respira- the upper and lower respiratory tract. The general population tory tract are a reflection of its vulnerability to micro- commonly experiences upper respiratory tract infections, bial attack. Acquisition of microbial pathogens is which are often seen in general practice. Lower respiratory tract infections are less common but are more likely to cause serious illness and death. Diagnosis and specific chemotherapy of respiratory tract infections present a particular challenge to both the clinician and the laboratory staff. -

The Physiology of Cardiopulmonary Resuscitation
Society for Critical Care Anesthesiologists Section Editor: Avery Tung E REVIEW ARTICLE CME The Physiology of Cardiopulmonary Resuscitation Keith G. Lurie, MD, Edward C. Nemergut, MD, Demetris Yannopoulos, MD, and Michael Sweeney, MD * † ‡ § Outcomes after cardiac arrest remain poor more than a half a century after closed chest cardiopulmonary resuscitation (CPR) was first described. This review article is focused on recent insights into the physiology of blood flow to the heart and brain during CPR. Over the past 20 years, a greater understanding of heart–brain–lung interactions has resulted in novel resuscitation methods and technologies that significantly improve outcomes from cardiac arrest. This article highlights the importance of attention to CPR quality, recent approaches to regulate intrathoracic pressure to improve cerebral and systemic perfusion, and ongo- ing research related to the ways to mitigate reperfusion injury during CPR. Taken together, these new approaches in adult and pediatric patients provide an innovative, physiologically based road map to increase survival and quality of life after cardiac arrest. (Anesth Analg 2016;122:767–83) udden cardiac arrest remains a leading cause of pre- during CPR, and new approaches to reduce injury associ- hospital and in-hospital death.1 Efforts to resuscitate ated with reperfusion.3,5–8,10–48 Given the debate surrounding patients after cardiac arrest have preoccupied scien- what is known, what we think we know, and what remains S 1,2 tists and clinicians for decades. However, the majority unknown about resuscitation science, this article also pro- of patients are never successfully resuscitated.1,3–5 Based vides some contrarian and nihilistic points of view. -

CARBON MONOXIDE: the SILENT KILLER Information You Should
CARBON MONOXIDE: THE SILENT KILLER Information You Should Know Carbon monoxide is a silent killer that can lurk within fossil fuel burning household appliances. Many types of equipment and appliances burn different types of fuel to provide heat, cook, generate electricity, power vehicles and various tools, such as chain saws, weed eaters and leaf blowers. When these units operate properly, they use fresh air for combustion and vent or exhaust carbon dioxide. When fresh air is restricted, through improper ventilation, the units create carbon monoxide, which can saturate the air inside the structure. Carbon Monoxide can be lethal when accidentally inhaled in concentrated doses. Such a situation is referred to as carbon monoxide poisoning. This is a serious condition that is a medical emergency that should be taken care of right away. What Is It? Carbon monoxide, often abbreviated as CO, is a gas produced by burning fossil fuel. What makes it such a silent killer is that it is odorless and colorless. It is extremely difficult to detect until the body has inhaled a detrimental amount of the gas, and if inhaled in high concentrations, it can be fatal. Carbon monoxide causes tissue damage by blocking the body’s ability to absorb enough oxygen. In fact, poisoning from this gas is one of the leading causes of unintentional death from poison. Common Sources of CO Kerosene or fuel-based heaters Fireplaces Gasoline powered equipment and generators Charcoal grills Automobile exhaust Portable generators Tobacco smoke Chimneys, furnaces, and boilers Gas water heaters Wood stoves and gas stoves Properly installed and maintained appliances are safe and efficient. -

Hyperoxia-Induced Pulmonary Vascular and Lung Abnormalities in Young Rats and Potential for Recovery
003 1-399818511910-1059$02.00/0 PEDIATRIC RESEARCH Vol. 19, No. 10, 1985 Copyright O 1985 International Pediatric Research Foundation, Inc. Prinfed in U.S.A. Hyperoxia-Induced Pulmonary Vascular and Lung Abnormalities in Young Rats and Potential for Recovery WENDY LEE WILSON, MICHELLE MULLEN, PETER M. OLLEY, AND MARLENE RABINOVITCH Departments of Cardiology and Pathology, Research Institute, The Hospital for Sick Children and Departments of Pediatrics and Pathology, University of Toronto, Toronto, Canada ABSTRACT. We carried out morphometric studies to The newborn infant, especially the premature with hyaline assess the effects of increasing durations of hyperoxic membrane disease, may require prolonged high oxygen therapy. exposure on the developing rat lung and to evaluate the Hyperoxia is damaging to lung tissue presumably because the potential for new growth and for regression of structural relatively inert O2 molecule undergoes univalent reduction to abnormalities on return to room air. From day 10 of life form highly reactive free radicals which are cytotoxic (1). Some Sprague-Dawley rats were either exposed to hyperoxia protection is afforded by the antioxidant enzyme systems of the (0.8F102) for 2-8 wk or were removed after 2 wk and lungs, superoxide dismutase, catalase, and glutathione peroxidase allowed to "recover" in room air for 2-6 wk. Litter mates (2-5) but the induction of these enzymes is often insufficient to maintained in room air served as age matched controls. prevent tissue damage. In the clinical setting it has been difficult Every 2 wk experimental and control rats from each group to separate the role of oxygen toxicity from other variables such were weighed and killed. -

Effects of Anaesthesia Techniques and Drugs on Pulmonary Function
Review Article Effects of anaesthesia techniques and drugs on pulmonary function Address for correspondence: Vijay Saraswat Dr. Vijay Saraswat, Department of Anaesthesiology, Apollo Hospitals, Nashik, Maharashtra, India Apollo Hospitals, Nashik, Maharashtra, India. E‑mail: drvsaraswat@gmail. ABSTRACT com The primary task of the lungs is to maintain oxygenation of the blood and eliminate carbon dioxide through the network of capillaries alongside alveoli. This is maintained by utilising ventilatory reserve capacity and by changes in lung mechanics. Induction of anaesthesia impairs pulmonary functions by the loss of consciousness, depression of reflexes, changes in rib cage and haemodynamics. All drugs used during anaesthesia, including inhalational agents, affect pulmonary functions directly by acting on respiratory system or indirectly through their actions on other systems. Volatile anaesthetic agents have more pronounced effects on pulmonary functions compared to intravenous induction agents, leading to hypercarbia and hypoxia. The posture of the patient also leads to major changes in pulmonary functions. Anticholinergics and neuromuscular blocking agents have little effect. Analgesics and Access this article online sedatives in combination with volatile anaesthetics and induction agents may exacerbate Website: www.ijaweb.org their effects. Since multiple agents are used during anaesthesia, ultimate effect may be DOI: 10.4103/0019‑5049.165850 different from when used in isolation. Literature search was done using MeSH key words ‘anesthesia’, -

Respiratory System
Respiratory System 1 Respiratory System 2 Respiratory System 3 Respiratory System 4 Respiratory System 5 Respiratory System 6 Respiratory System 7 Respiratory System 8 Respiratory System 9 Respiratory System 10 Respiratory System 11 Respiratory System • Pulmonary Ventilation 12 Respiratory System 13 Respiratory System 14 Respiratory System • Measuring of Lung Function œ Compliance œ the ease at which the lungs and thoracic wall can be expanded œ if reduced it is more difficult to inflate the lungs œ causes: • Damaged lung tissue • Fluid within lung tissue • Decrease in pulmonary surfactant • Anything that impedes lung expansion or contraction œ Respiratory Volumes and Capacities will be covered in Lab œ 15 Respiratory System • Exchange of Oxygen and Carbon Dioxide œ Charles‘ Law œ the volume of a gas is directly proportional to the absolute temperature, assuming the pressure remains constant As gases enter the lung they warm and expand, increasing lung volume œ Dalton‘s Law œ each gas of a mixture of gases exerts its own pressure as if all the other gases were not present œ Henry‘s Law œ the quantity of a gas that will dissolve in a liquid is proportional to the partial pressure of the gas and its solubility coefficient, when the temperature remains constant 16 Respiratory System • External and Internal Respiration 17 Respiratory System • Transport of Oxygen and Carbon Dioxide by the Blood œ Oxygen Transport • 1.5% dissolved in plasma • 98.5% carried with Hbinside of RBC‘s as oxyhemoglobin œ Hbœ made up of protein portion called the globinportion -
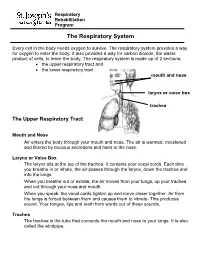
The Respiratory System
Respiratory Rehabilitation Program The Respiratory System Every cell in the body needs oxygen to survive. The respiratory system provides a way for oxygen to enter the body. It also provides a way for carbon dioxide, the waste product of cells, to leave the body. The respiratory system is made up of 2 sections: the upper respiratory tract and the lower respiratory tract mouth and nose larynx or voice box trachea The Upper Respiratory Tract Mouth and Nose Air enters the body through your mouth and nose. The air is warmed, moistened and filtered by mucous secretions and hairs in the nose. Larynx or Voice Box The larynx sits at the top of the trachea. It contains your vocal cords. Each time you breathe in or inhale, the air passes through the larynx, down the trachea and into the lungs. When you breathe out or exhale, the air moves from your lungs, up your trachea and out through your nose and mouth. When you speak, the vocal cords tighten up and move closer together. Air from the lungs is forced between them and causes them to vibrate. This produces sound. Your tongue, lips and teeth form words out of these sounds. Trachea The trachea is the tube that connects the mouth and nose to your lungs. It is also called the windpipe. The Lower Respiratory Tract Inside Lungs Outside Lungs bronchial tubes alveoli diaphragm (muscle) Bronchial Tubes The trachea splits into 2 bronchial tubes in your lungs. These are called the left bronchus and right bronchus. The bronchus tubes keep branching off into smaller and smaller tubes called bronchi. -

RESPIRATORY TRACT INFECTIONS Peter Zajac, DO, FACOFP, Author Amy J
OFP PATIENT EDUCATION HANDOUT RESPIRATORY TRACT INFECTIONS Peter Zajac, DO, FACOFP, Author Amy J. Keenum, DO, PharmD, Editor • Ronald Januchowski, DO, FACOFP, Health Literacy Editor HOME MANAGEMENT INCLUDES: • Drinking plenty of clear fuids and rest. Vitamin-C may help boost your immune system. Over-the-counter pain relievers such as acetaminophen and ibuprofen can be helpful for fevers and to ease any aches. Saline (salt) nose drops, lozenges, and vapor rubs can also help symptoms when used as directed by your physician. • A cool mist humidifer can make breathing easier by thinning mucus. • If you smoke, you should try to stop smoking for good! Avoid second-hand smoking also. • In most cases, antibiotics are not recommended because they are only effective if bacteria caused the infection. • Other treatments, that your Osteopathic Family Physician may prescribe, include Osteopathic Manipulative Therapy (OMT). OMT can help clear mucus, Respiratory tract infections are any relieve congestion, improve breathing and enhance comfort, relaxation, and infection that affect the nose, sinuses, immune function. and throat (i.e. the upper respiratory tract) or airways and lungs (i.e. the • Generally, the symptoms of a respiratory tract infection usually pass within lower respiratory tract). Viruses are one to two weeks. the main cause of the infections, but • To prevent spreading infections, sneeze into the arm of your shirt or in a tissue. bacteria can cause some. You can Also, practice good hygiene such as regularly washing your hands with soap and spread the infection to others through warm water. Wipe down common surfaces, such as door knobs and faucet handles, the air when you sneeze or cough. -

THE 6 MAJOR BODY SYSTEMS and How They Interact with Each Other to Keep the “Body Machine” Alive and Working Well
THE 6 MAJOR BODY SYSTEMS And how they interact with each other to keep the “body machine” alive and working well. CIRCULATORY SYSTEM / CARDIOVASCULAR SYSTEM PRIMARY PURPOSE: transport blood throughout the body by circulating PRIMARY ORGANS/PARTS: Heart, blood vessels (arteries, veins, capillaries) (1) Transports/carries nutrients and oxygen through the blood to most parts of the body (2) Transports/carries waste in cells and carbon-dioxide (CO2) away from the parts: (a) Cell waste goes to the kidneys for filter and disposal (b) Carbon-dioxide (CO2) goes to the lungs to exhale (breathe out) Kidneys and Lungs have a close relationship with Cardiovascular system Kidneys: filter through blood to take out the waste and get it eventually out of the body Lungs: breathes in oxygen and gives it to the blood for Circulatory system to carry throughout the body; and takes unneeded carbon-dioxide (CO2) from the blood and breathes that out. Circulatory/Cardiovascular System through the blood to most parts of the body provides nutrients and oxygen which is needed for our bodies to have ENERGY! RESPIRATORY SYSTEM PRIMARY PURPOSE: Breathing - taking in Oxygen, pushing out Carbon-Dioxide (CO2) PRIMARY ORGANS: Lungs, trachea (tube going from lungs to nose/mouth) (1) Inhales (breathes in) Oxygen - good for the body - gives it to the Circulatory System to be transported throughout the body through the blood. (2) Exhales (breathes out) Carbon-Dioxide (CO2) - lungs get this gas from the blood (Circ. Sys.) and pushes it out of the body DIGESTIVE SYSTEM PRIMARY PURPOSE: take in food; break down food into nutrients (good) and waste (unneeded) PRIMARY ORGANS: Stomach, large and small intestines, esophagus (tube from stomach to mouth) (1) Digestive System gets nutrients (good) from food and hands it over to the blood and Circulatory System then carries those nutrients where they need to go. -

Respiratory Tract Infections in the Tropics
9.1 CHAPTER 9 Respiratory Tract Infections in the Tropics Tim J.J. Inglis 9.1 INTRODUCTION Acute respiratory infections are the single most common infective cause of death worldwide. This is also the case in the tropics, where they are a major cause of death in children under five. Bacterial pneumonia is particularly common in children in the tropics, and is more often lethal. Pulmonary tuberculosis is the single most common fatal infection and is more prevalent in many parts of the tropics due to a combination of endemic HIV infection and widespread poverty. A lack of diagnostic tests, limited access to effective treatment and some traditional healing practices exacerbate the impact of respiratory infection in tropical communities. The rapid urbanisation of populations in the tropics has increased the risk of transmitting respiratory pathogens. A combination of poverty and overcrowding in the peri- urban zones of rapidly expanding tropical cities promotes the epidemic spread of acute respiratory infection. PART A. INFECTIONS OF THE LOWER RESPIRATORY TRACT 9.2 PNEUMONIA 9.2.1 Frequency Over four million people die from acute respiratory infection per annum, mostly in developing countries. There is considerable overlap between these respiratory deaths and deaths due to tuberculosis, the single commonest fatal infection. The frequency of acute respiratory infection differs with location due to host, pathogen and environmental factors. Detailed figures are difficult to find and often need to be interpreted carefully, even for tuberculosis where data collection is more consistent. But in urban settings the enormity of respiratory infection is clearly evident. Up to half the patients attending hospital outpatient departments in developing countries have an acute respiratory infection. -

Oxygen Toxicity in Neonatal Rats: the Effect of Endotoxin Treatment on Survival During and Post-02 Exposure
0031-3998/87/2102-0 I 09$02.00/0 PEDIATRIC RESEARCH Vol. 21. No.2, 1987 Copyright© 1987 International Pediatric Research Foundation, Inc. Printed in U.S.A. Oxygen Toxicity in Neonatal Rats: The Effect of Endotoxin Treatment on Survival during and post-02 Exposure LEE FRANK Department ofMedicine, Pulmonary Research Labora10ry, University of Miami School of Medicine, Miami, Florida 33136 ABSTRACT. Neonatal rats were treated with low doses of monary 0 2 toxicity (I, 2). However, there are unique pulmonary bacterial lipopolysaccharide (endotoxin) to test for a pro complications associated with prolonged hyperoxic exposure in tective effect of endotoxin against 02 toxicity and the the neonatal animal which could importantly influence both severe inhibition of normal lung development which occurs short and long-term survival post-02 exposure. These complica during prolonged exposure to hyperoxia. The rationale for tions relate to the known inhibitory action of hyperoxia on lung the prophylactic use of endotoxin included its marked biosynthetic processes (1, 3, 4), and the morphological conse protective effect against pulmonary 0 2 toxicity in adult quences this inhibitory action has on the rapidly growing and rats and its lung growth-promoting effect in experimental differentiating newborn (animal) lung. Several studies have now pulmonary stress models. Neonatal rats (4-5 days old) demonstrated that early postnatal exposure to hyperoxia is as survived a 14-day exposure to >95% 0 2 equally well sociated with marked inhibition of the normal septation process whether treated with saline (39/51 = 76%) or with endo by which the large saccular airspaces characteristic of the new toxin (41/51 = 80%).