The Ecology of an Hawaiian Coral Reef
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Hermatypic Coral Fauna of Subtropical Southeast Africa: a Checklist!
Pacific Science (1996), vol. 50, no. 4: 404-414 © 1996 by University of Hawai'i Press. All rights reserved Hermatypic Coral Fauna of Subtropical Southeast Africa: A Checklist! 2 BERNHARD RrnGL ABSTRACT: The South African hermatypic coral fauna consists of 96 species in 42 scleractinian genera, one stoloniferous octocoral genus (Tubipora), and one hermatypic hydrocoral genus (Millepora). There are more species in southern Mozambique, with 151 species in 49 scleractinian genera, one stolo niferous octocoral (Tubipora musica L.), and one hydrocoral (Millepora exaesa [Forskal)). The eastern African coral faunas of Somalia, Kenya, Tanzania, Mozambique, and South Africa are compared and Southeast Africa dis tinguished as a biogeographic subregion, with six endemic species. Patterns of attenuation and species composition are described and compared with those on the eastern boundaries of the Indo-Pacific in the Pacific Ocean. KNOWLEDGE OF CORAL BIODIVERSITY in the Mason 1990) or taxonomically inaccurate Indo-Pacific has increased greatly during (Boshoff 1981) lists of the corals of the high the past decade (Sheppard 1987, Rosen 1988, latitude reefs of Southeast Africa. Sheppard and Sheppard 1991 , Wallace and In this paper, a checklist ofthe hermatypic Pandolfi 1991, 1993, Veron 1993), but gaps coral fauna of subtropical Southeast Africa, in the record remain. In particular, tropical which includes the southernmost corals of and subtropical subsaharan Africa, with a Maputaland and northern Natal Province, is rich and diverse coral fauna (Hamilton and evaluated and compared with a checklist of Brakel 1984, Sheppard 1987, Lemmens 1993, the coral faunas of southern Mozambique Carbone et al. 1994) is inadequately docu (Boshoff 1981). -

FDM 2017 Coral Species Reef Survey
Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific Final ® FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT Dr. Jessica Carilli, SSC Pacific Mr. Stephen H. Smith, SSC Pacific Mr. Donald E. Marx Jr., SSC Pacific Dr. Leslie Bolick, SSC Pacific Dr. Douglas Fenner, NOAA August 2018 Prepared for U.S. Navy Pacific Fleet Commander Pacific Fleet 250 Makalapa Drive Joint Base Pearl Harbor Hickam Hawaii 96860-3134 Space and Naval Warfare Systems Center Pacific Technical Report number 18-1079 Distribution Statement A: Unlimited Distribution 1 Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Service, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington, DC 20503. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 08-2018 Monitoring report September 2017 - October 2017 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT 5b. -

Bacterial Profiling of White Plague Disease Across Corals and Oceans
Molecular Ecology (2014) 23, 965–974 doi: 10.1111/mec.12638 Bacterial profiling of White Plague Disease across corals and oceans indicates a conserved and distinct disease microbiome CORNELIA RODER,* CHATCHANIT ARIF,* CAMILLE DANIELS,* ERNESTO WEIL† and CHRISTIAN R. VOOLSTRA* *Red Sea Research Center, King Abdullah University of Science and Technology, 23955 Thuwal, Saudi Arabia, †Department of Marine Sciences, University of Puerto Rico, PO BOX 9000, Mayaguez, Puerto Rico 00680, USA Abstract Coral diseases are characterized by microbial community shifts in coral mucus and tissue, but causes and consequences of these changes are vaguely understood due to the complexity and dynamics of coral-associated bacteria. We used 16S rRNA gene microarrays to assay differences in bacterial assemblages of healthy and diseased colo- nies displaying White Plague Disease (WPD) signs from two closely related Caribbean coral species, Orbicella faveolata and Orbicella franksi. Analysis of differentially abun- dant operational taxonomic units (OTUs) revealed strong differences between healthy and diseased specimens, but not between coral species. A subsequent comparison to data from two Indo-Pacific coral species (Pavona duerdeni and Porites lutea) revealed distinct microbial community patterns associated with ocean basin, coral species and health state. Coral species were clearly separated by site, but also, the relatedness of the underlying bacterial community structures resembled the phylogenetic relationship of the coral hosts. In diseased samples, bacterial richness increased and putatively opportunistic bacteria were consistently more abundant highlighting the role of oppor- tunistic conditions in structuring microbial community patterns during disease. Our comparative analysis shows that it is possible to derive conserved bacterial footprints of diseased coral holobionts that might help in identifying key bacterial species related to the underlying etiopathology. -
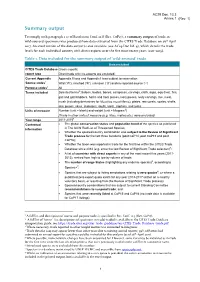
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

Divergent Evolutionary Histories of DNA Markers in a Hawaiian Population of the Coral Montipora Capitata Hollie M
University of Rhode Island DigitalCommons@URI Biological Sciences Faculty Publications Biological Sciences 2017 Divergent Evolutionary Histories of DNA Markers in a Hawaiian Population of the Coral Montipora capitata Hollie M. Putnam University of Rhode Island, [email protected] Diane K. Adams See next page for additional authors Creative Commons License Creative Commons License This work is licensed under a Creative Commons Attribution 4.0 License. Follow this and additional works at: https://digitalcommons.uri.edu/bio_facpubs Citation/Publisher Attribution Putnam, H. M., Adams, D. K., Zelzion, E., Wagner, N. E., Qiu, H., Mass, T., ... Bhattacharya, D. (2017). Divergent evolutionary histories of DNA markers in a Hawaiian population of the coral Montipora capitata. PeerJ, 5, e3319. doi: 10.7717/peerj.3319. This Article is brought to you for free and open access by the Biological Sciences at DigitalCommons@URI. It has been accepted for inclusion in Biological Sciences Faculty Publications by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. Authors Hollie M. Putnam, Diane K. Adams, Ehud Zelzion, Nicole E. Wagner, Huan Qiu, Tali Mass, Paul G. Falkowski, Ruth D. Gates, and Debashish Bhattacharya This article is available at DigitalCommons@URI: https://digitalcommons.uri.edu/bio_facpubs/85 Divergent evolutionary histories of DNA markers in a Hawaiian population of the coral Montipora capitata Hollie M. Putnam1,2,*, Diane K. Adams3,*, Ehud Zelzion4, Nicole E. Wagner4, Huan Qiu4, -

Successive Bleaching Events Cause Mass Coral Mortality in Guam, Micronesia
Successive bleaching events cause mass coral mortality in Guam, Micronesia L. J. Raymundo, D. Burdick, W. C. Hoot, R. M. Miller, V. Brown, T. Reynolds, J. Gault, J. Idechong, J. Fifer & A. Williams Coral Reefs Journal of the International Coral Reef Society ISSN 0722-4028 Coral Reefs DOI 10.1007/s00338-019-01836-2 1 23 1 23 Coral Reefs https://doi.org/10.1007/s00338-019-01836-2 REPORT Successive bleaching events cause mass coral mortality in Guam, Micronesia 1 1 2 1 3 L. J. Raymundo • D. Burdick • W. C. Hoot • R. M. Miller • V. Brown • 1 1 1 1,4 1 T. Reynolds • J. Gault • J. Idechong • J. Fifer • A. Williams Received: 15 October 2018 / Accepted: 17 June 2019 Ó Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The reefs of Guam, a high island in the Western Preliminary evidence suggests that some coral species are at Pacific, were impacted by an unprecedented succession of high risk of extirpation from Guam’s waters. In light of extreme environmental events beginning in 2013. Elevated predictions of the near-future onset of severe annual SSTs induced severe island-wide bleaching in 2013, 2014, bleaching, and the possibility that the events of 2013–2017 2016, and 2017. Additionally, a major ENSO event triggered may signal the early arrival of these conditions, the persis- extreme low tides beginning in 2014 and extending through tence of Guam’s current reef assemblages is in question. 2015, causing additional coral mortality from subaerial Here, we present detailed documentation of ongoing changes exposure on shallow reef flat platforms. -

On Shallow Reefs of the Northwestern Hawaiian Islands. Part 1: Species and Distribution1
2000-2002 Rapid Ecological Assessment of Corals (Anthozoa) on Shallow Reefs of the Northwestern Hawaiian Islands. Part 1: Species and Distribution1 James E. Maragos, 2 Donald C. Potts, 3 Greta Aeby, 4 Dave Gulko, 4 Jean Kenyon,S Daria Siciliano, 3 and Dan VanRavenswaay6 Abstract: Rapid Ecological Assessment (REA) surveys at 465 sites on 11 reefs in the Northwestern Hawaiian Islands (NWHI) inventoried coral species, their relative abundances, and their distributions during 2000-2002. Surveys (462) around the 10 islands were in depths of ~20 m, and three surveys on the sub merged Raita Bank were in depths of 30-35 m. Data from 401 REA sites met criteria for quantitative analysis. Results include 11 first records for stony coral species in the Hawaiian Archipelago and 29 range extensions to the NWHI. Several species may be new to science. There are now 57 stony coral species known in the shallow subtropical waters ofthe NWHI, similar to the 59 shallow and deep-water species known in the better-studied and more tropical main Hawaiian Islands. Coral endemism is high in the NWHI: 17 endemic species (30%) account for 37-53% of the abundance of stony corals on each reef of the NWHI. Three genera (Montipora, Porites, Pocillopora) contain 15 of the 17 en demic species and most of the endemic abundance. Seven Acropora species are now known from the central NWHI despite their near absence from the main Hawaiian Islands. Coral abundance and diversity are highest at the large, open atolls of the central NWHI (French Frigate, Maro, Lisianski) and decline gradually through the remaining atolls to the northwest (Pearl and Hermes, Midway, and Kure). -

Persistence of Corals in Marginal Habitats: the Role of the Environment, and Symbiont Diversity and Ecophysiology
Persistence of corals in marginal habitats: the role of the environment, and symbiont diversity and ecophysiology Laura Caroline Wicks A thesis submitted to Victoria University of Wellington in fulfillment of the requirements for the degree of Doctor of Philosophy in Science 2009 Abstract Abstract Many corals live in marginal habitats, close to their survival thresholds of water temperature, light penetration and aragonite saturation. Living under these highly variable and extreme conditions is likely facilitated by specific physiological adaptations and/or the presence of unique species of coral and their symbionts but data on these factors are limited. The specific objectives of the study were to: (1) examine the diversity and distribution patterns of corals in marginal environments, (2) investigate the diversity, distribution patterns and host specificity of symbionts in corals in marginal environments, (3) assess the influence of environmental variables on host and symbiont distribution in marginal environments, in comparison to ‘optimal’ environments, and (4) examine the physiological responses to changing environmental conditions and stress of corals and their symbionts in marginal environments. Surveys of coral community patterns were conducted at the Kermadec Islands (KI), New Zealand, and Palmyra Atoll, USA, with local scale environmental parameters (i.e. wave exposure and sedimentation) found to control the diversity and distribution of the coral communities. Symbiodinium types were identified to sub- cladal level in a range of coral species at each of the survey sites, using ITS2-DGGE. A high diversity of C type symbionts (19 types in 13 host genera), and reduced host specificity was observed at the high latitude site of Lord Howe Island (LHI), Australia, with similarly high diversity at the KI (10 types in 9 genera). -

Abundance, Distribution and New Records of Scleractinian Corals at Barrow Island and Southern Montebello Islands, Pilbara (Offshore) Bioregion
Journal of the Royal Society of Western Australia, 95: 155–165, 2012 Abundance, distribution and new records of scleractinian corals at Barrow Island and Southern Montebello Islands, Pilbara (Offshore) Bioregion Z T RICHARDS 1* & N L ROSSER 2† 1 Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986, Australia. 2 RPS Environment, 38 Station Street, Subiaco, WA 6008, Australia. * Corresponding Author: ! [email protected] † Current address: School of Animal Biology, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia. The local abundance and distribution of scleractinian corals were documented near Barrow Island in the Pilbara (Offshore) Bioregion, Western Australia. Using a standard rapid ecological assessment method we recorded 204 species from 51 genera and as a result of this study we extend the known distribution range of 15 species. We find a high diversity of habitat types promotes high species richness, particularly among Acropora species. Our results confirm the existence of a unique suite of coral species in the Pilbara that is not recorded in the Oceanic Shoals (Offshore) or Pilbara (Nearshore) Bioregions. The Pilbara has a rich coral fauna that is often overlooked and the Barrow/ Montebello Islands group may provide a high latitude refuge for some coral species including 39 species that are listed as Vulnerable on the IUCN red list of threatened species. KEYWORDS: biodiversity, corals, demography, hermatypic, IUCN, local extinction. INTRODUCTION For species to migrate south from tropical locations in Western Australia it is necessary for them to pass The status of coral-reef ecosystems is closely related to through the Pilbara region.