Bacterial Profiling of White Plague Disease Across Corals and Oceans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

UC Merced UC Merced Electronic Theses and Dissertations
UC Merced UC Merced Electronic Theses and Dissertations Title Deep Amplicon Sequencing Quantitatively Detected Mixed Community Assemblages of Symbiodinium in Orbicella faveolata and Orbicella franksi Permalink https://escholarship.org/uc/item/31n4975j Author Green, Elizabeth Publication Date 2014 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, MERCED Deep Amplicon Sequencing Quantitatively Detected Mixed Community Assemblages of Symbiodinium in Orbicella faveolata and Orbicella franksi THESIS submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Quantitative and Systems Biology by Elizabeth A. Green Committee in charge: David Ardell, chair Miriam Barlow Mónica Medina Michele Weber 2014 © Elizabeth A. Green, 2014 All rights reserved The thesis of Elizabeth A. Green is approved, and it is acceptable in quality and form for publication on microfilm and electronically: Miriam Barlow Mónica Medina Michele Weber David Ardell Chair University of California, Merced 2014 iii Dedication This thesis is dedicated to my loving and supportive husband, Colten Green. iv Table of Contents Page SIGNATURE PAGE ……………………….…………………………………… iii LIST OF FIGURES ……………………………………………………………... vi LIST OF TABLES ………………………………………………………………. vii ACKNOWLEDGEMENTS ……………………………………………………… viii ABSTRACT ……………………………………………………………………… ix INTRODUCTION ………………………………………………………………… 1 METHODS ………………………………………………………………………… 6 RESULTS …………………………………………………………………………. -

Marine Ecology Progress Series 506:129
Vol. 506: 129–144, 2014 MARINE ECOLOGY PROGRESS SERIES Published June 23 doi: 10.3354/meps10808 Mar Ecol Prog Ser FREEREE ACCESSCCESS Long-term changes in Symbiodinium communities in Orbicella annularis in St. John, US Virgin Islands Peter J. Edmunds1,*, Xavier Pochon2,3, Don R. Levitan4, Denise M. Yost2, Mahdi Belcaid2, Hollie M. Putnam2, Ruth D. Gates2 1Department of Biology, California State University, 18111 Nordhoff Street, Northridge, CA 91330-8303, USA 2Hawaii Institute of Marine Biology, University of Hawaii, PO Box 1346, Kaneohe, HI 96744, USA 3Environmental Technologies, Cawthron Institute, 98 Halifax Street East, Private Bag 2, Nelson 7042, New Zealand 4Department of Biological Science, Florida State University, Tallahassee, FL 32306-4295, USA ABSTRACT: Efforts to monitor coral reefs rarely combine ecological and genetic tools to provide insight into the processes driving patterns of change. We focused on a coral reef at 14 m depth in St. John, US Virgin Islands, and used both sets of tools to examine 12 colonies of Orbicella (for- merly Montastraea) annularis in 2 photoquadrats that were monitored for 16 yr and sampled genetically at the start and end of the study. Coral cover and colony growth were assessed annu- ally, microsatellites were used to genetically identify coral hosts in 2010, and their Symbiodinium were genotyped using chloroplastic 23S (cloning) and nuclear ITS2 (cloning and pyrosequencing) in 1994 and 2010. Coral cover declined from 40 to 28% between 1994 and 2010, and 3 of the 12 sampled colonies increased in size, while 9 decreased in size. The relative abundance of Symbio- dinium clades varied among corals over time, and patterns of change differed between photo- quadrats but not among host genotypes. -

FDM 2017 Coral Species Reef Survey
Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific Final ® FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT Dr. Jessica Carilli, SSC Pacific Mr. Stephen H. Smith, SSC Pacific Mr. Donald E. Marx Jr., SSC Pacific Dr. Leslie Bolick, SSC Pacific Dr. Douglas Fenner, NOAA August 2018 Prepared for U.S. Navy Pacific Fleet Commander Pacific Fleet 250 Makalapa Drive Joint Base Pearl Harbor Hickam Hawaii 96860-3134 Space and Naval Warfare Systems Center Pacific Technical Report number 18-1079 Distribution Statement A: Unlimited Distribution 1 Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Service, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington, DC 20503. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 08-2018 Monitoring report September 2017 - October 2017 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT 5b. -

Coral-Associated Bacteria Demonstrate Phylosymbiosis and Cophylogeny
ARTICLE DOI: 10.1038/s41467-018-07275-x OPEN Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny F. Joseph Pollock1, Ryan McMinds 2, Styles Smith1, David G. Bourne3,4, Bette L. Willis3,5, Mónica Medina 1,6, Rebecca Vega Thurber2 & Jesse R. Zaneveld7 Scleractinian corals’ microbial symbionts influence host health, yet how coral microbiomes assembled over evolution is not well understood. We survey bacterial and archaeal 1234567890():,; communities in phylogenetically diverse Australian corals representing more than 425 million years of diversification. We show that coral microbiomes are anatomically compartmenta- lized in both modern microbial ecology and evolutionary assembly. Coral mucus, tissue, and skeleton microbiomes differ in microbial community composition, richness, and response to host vs. environmental drivers. We also find evidence of coral-microbe phylosymbiosis, in which coral microbiome composition and richness reflect coral phylogeny. Surprisingly, the coral skeleton represents the most biodiverse coral microbiome, and also shows the stron- gest evidence of phylosymbiosis. Interactions between bacterial and coral phylogeny sig- nificantly influence the abundance of four groups of bacteria–including Endozoicomonas-like bacteria, which divide into host-generalist and host-specific subclades. Together these results trace microbial symbiosis across anatomy during the evolution of a basal animal lineage. 1 Department of Biology, Pennsylvania State University, 208 Mueller Lab, University Park, State College, PA 16802, USA. 2 Department of Microbiology, Oregon State University, 226 Nash Hall, Corvallis, OR 97331, USA. 3 College of Science and Engineering, James Cook University, Townsville, QLD 4811, Australia. 4 Australian Institute of Marine Science, Townsville, QLD 4810, Australia. 5 ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD 4811, Australia. -

Protected Species Order 2015
Protected Species Order 2015 August 2015 GOVERNMENT OF BERMUDA MINISTRY OF HEALTH, SENIORS AND ENVIRONMENT Department of Conservation Services Protected Species Order 2015 – Protected Species Act 2003 2015 Bermuda and the surrounding reef platform, 1998 Bermuda and the surrounding reef platform, 1998 Protected Species Order 2015 – Protected Species Act 2003 Table of Contents 1.0. Introduction ................................................................................................................................................................................................ 1 Purpose of legislation ...................................................................................................................................................................................... 2 Goal ................................................................................................................................................................................................................. 2 Objectives ........................................................................................................................................................................................................ 2 How species are nominated ............................................................................................................................................................................. 2 Levels of protection for protected species ...................................................................................................................................................... -
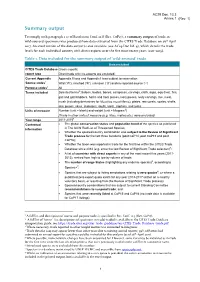
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

Growth Rates of Porites Astreoides and Orbicella Franksi in Mesophotic Habitats Surrounding St
Growth rates of Porites astreoides and Orbicella franksi in mesophotic habitats surrounding St. Thomas Sarah H. Groves1,5,*, Daniel M. Holstein2, Ian C. Enochs3,4, Graham Kolodzeij3, Derek P. Manzello3, Marilyn E. Brandt5, Tyler B. Smith5 1. National Centers for Coastal Ocean Science Marine Spatial Ecology Division, National Oceanic and Atmospheric Administration, 101 Pivers Island Rd., Beaufort, NC 28516, USA 2. Duke University Marine Laboratory, Nicholas School of the Environment, Duke University, 135 Duke Marine Lab Rd., Beaufort, NC 28516 3.Atlantic Oceanographic and Meteorological Laboratories (AOML), NOAA, 4301 Rickenbacker Cswy., Miami, FL 33149, USA 4. Cooperative Institute for Marine and Atmospheric Studies, Rosenstiel School of Marine and Atmospheric Science, University of Miami, 4600 Rickenbacker Cswy., Miami, FL 33149, USA 5. Center for Marine and Environmental Studies, University of the Virgin Islands, 2 John Brewer’s Bay, St. Thomas, Virgin Islands 00802, USA *Communicating author: [email protected] Keywords Mesophotic coral ecosystems, Sclerochronology, Refugia, Shelf edge reefs, Orbicella spp. Abstract Mesophotic coral ecosystems (MCEs) are deep (>30 m), light-dependent communities that are abundant in many parts of the global ocean. MCEs are potentially connected to shallow reefs via larval exchange and may act as refuges for reef organisms. However, MCE coral community recovery after disturbance, and thus, community resilience, are poorly understood components of their capacity as refuges. To assess the potential for disturbance and growth to drive community structure on MCEs with differential biophysical conditions and coral communities, we collected colonies of Orbicella franksi and Porites astreoides and used computerized tomography to quantify calcification. The divergence of coral growth rates in MCEs with different environmental conditions may be species specific; habitat-forming O. -

Successive Bleaching Events Cause Mass Coral Mortality in Guam, Micronesia
Successive bleaching events cause mass coral mortality in Guam, Micronesia L. J. Raymundo, D. Burdick, W. C. Hoot, R. M. Miller, V. Brown, T. Reynolds, J. Gault, J. Idechong, J. Fifer & A. Williams Coral Reefs Journal of the International Coral Reef Society ISSN 0722-4028 Coral Reefs DOI 10.1007/s00338-019-01836-2 1 23 1 23 Coral Reefs https://doi.org/10.1007/s00338-019-01836-2 REPORT Successive bleaching events cause mass coral mortality in Guam, Micronesia 1 1 2 1 3 L. J. Raymundo • D. Burdick • W. C. Hoot • R. M. Miller • V. Brown • 1 1 1 1,4 1 T. Reynolds • J. Gault • J. Idechong • J. Fifer • A. Williams Received: 15 October 2018 / Accepted: 17 June 2019 Ó Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The reefs of Guam, a high island in the Western Preliminary evidence suggests that some coral species are at Pacific, were impacted by an unprecedented succession of high risk of extirpation from Guam’s waters. In light of extreme environmental events beginning in 2013. Elevated predictions of the near-future onset of severe annual SSTs induced severe island-wide bleaching in 2013, 2014, bleaching, and the possibility that the events of 2013–2017 2016, and 2017. Additionally, a major ENSO event triggered may signal the early arrival of these conditions, the persis- extreme low tides beginning in 2014 and extending through tence of Guam’s current reef assemblages is in question. 2015, causing additional coral mortality from subaerial Here, we present detailed documentation of ongoing changes exposure on shallow reef flat platforms. -

On Shallow Reefs of the Northwestern Hawaiian Islands. Part 1: Species and Distribution1
2000-2002 Rapid Ecological Assessment of Corals (Anthozoa) on Shallow Reefs of the Northwestern Hawaiian Islands. Part 1: Species and Distribution1 James E. Maragos, 2 Donald C. Potts, 3 Greta Aeby, 4 Dave Gulko, 4 Jean Kenyon,S Daria Siciliano, 3 and Dan VanRavenswaay6 Abstract: Rapid Ecological Assessment (REA) surveys at 465 sites on 11 reefs in the Northwestern Hawaiian Islands (NWHI) inventoried coral species, their relative abundances, and their distributions during 2000-2002. Surveys (462) around the 10 islands were in depths of ~20 m, and three surveys on the sub merged Raita Bank were in depths of 30-35 m. Data from 401 REA sites met criteria for quantitative analysis. Results include 11 first records for stony coral species in the Hawaiian Archipelago and 29 range extensions to the NWHI. Several species may be new to science. There are now 57 stony coral species known in the shallow subtropical waters ofthe NWHI, similar to the 59 shallow and deep-water species known in the better-studied and more tropical main Hawaiian Islands. Coral endemism is high in the NWHI: 17 endemic species (30%) account for 37-53% of the abundance of stony corals on each reef of the NWHI. Three genera (Montipora, Porites, Pocillopora) contain 15 of the 17 en demic species and most of the endemic abundance. Seven Acropora species are now known from the central NWHI despite their near absence from the main Hawaiian Islands. Coral abundance and diversity are highest at the large, open atolls of the central NWHI (French Frigate, Maro, Lisianski) and decline gradually through the remaining atolls to the northwest (Pearl and Hermes, Midway, and Kure). -

Comparative Thermal Performance of Orbicella Franksi at Its Latitudinal Range Limits 2 Running Title: Comparative Thermal Performance of O
bioRxiv preprint doi: https://doi.org/10.1101/583294; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Comparative thermal performance of Orbicella franksi at its latitudinal range limits 2 Running title: Comparative thermal performance of O. franksi 3 4 Nyssa J. Silbiger*1, Gretchen Goodbody-Gringley2, John F. Bruno3, Hollie M. Putnam4 5 6 7 1Department of Biology, California State University, 18111 Nordhoff Street, Northridge, CA 8 91330-8303, USA 9 2Bermuda Institute of Ocean Sciences, 17 Biological Station, Ferry Reach, St.George's GE 01 10 Bermuda 11 3Department of Biology, The University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599- 12 3280 USA 13 4University of Rhode Island, Department of Biological Sciences, 120 Flagg Rd. Kingston, RI 14 02881 USA 15 16 17 18 * Corresponding Author 19 [email protected] 20 21 Keywords: Thermal performance curve, gross photosynthesis, dark respiration, calcification, 22 coral, climate change 23 24 25 Summary statement: We apply a thermal performance curve approach to a variety of fitness 26 related parameters in a reef building coral across its geographic range and various functions to 27 improve our understanding of the inherent variability in thermal tolerance. 28 29 30 31 1 bioRxiv preprint doi: https://doi.org/10.1101/583294; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.