Proton - Wikipedia Page 1 of 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

01 Freer.Pdf
John Henry Poynting First Prof Physics Birmingham 1880-1914 Rudolf Peierls 1907-1995 Birmingham 1937+ (holes in semiconductors) Marcus Laurence Elwin Oliphant 1901-2000 Birmingham 1937-1950 Otto Frisch (1904-1979) Bham 1939+ co-discover of 2H, 3H and 3He Role in first demonstration of nuclear fusion Got plans from Lawrence for Birmingham’s cyclotron John Bell 1928-1990 CPT 1951 Birmingham Luders-Pauli Thm Freeman-Dyson Tony Skyrme (1922-1987) Birmingham, 1949-51 Why are light nuclei so important – Understanding cutting edge theory ? [Otsuka et al., PRL 95 (05) 232502] Tensor correlations [Otsuka et al., PRL 97 (06) 162501] Neutron – proton interaction - - - + + + + - 8Be Ab initio type approaches (Greens Function Monte Carlo) 13 11 + 9 4 7 Ex [MeV] [MeV] Ex Ex 5 3 2+ 1 + -1 0 -1 4 9 14 19 24 Pieper and J(J+1) Wiringa, ANL 9 Ab initio no-core solutions for nuclear structure: Be P. Maris, C. Cockrell, M. Caprio and J.P. Vary Total density Proton - Neutron density Shows that one neutron provides a “ring ” cloud around two alpha clusters binding them together Chase Cockrell, ISU PhD student 12 C Chiral EFT on the lattice 32 S 24 Mg 9Be 9B PHYSICAL REVIEW C 86 , 057306 (2012), PHYSICAL REVIEW C 86 , 014312 (2012) 10 Be 12 C 10 C Characterization of 10 Be * Gas inlet LAMP array Window (2.5 um mylar) LAMP 6He LEDA LAMP LEDA Collimator M. Freer, et al. PRL 2006 Test of method- measure 10.36 MeV 4 + resonance In 16 O ( 12 C+ α) LAMP LEDA LAMP LEDA Resonances in 16 O E( 12 C)=16 MeV Singles Coincidence 10 10 4He Energy Energy (MeV) (MeV) 12 C 0 0 8 24 8 24 Angle Angle Gas pressure = 100 Torr LAMP LEDA LAMP 4 Energy ( 12 C) LEDA θcm ( He) 180 0 Energy ( 4He) 12 180 θcm ( C) Resonance analysis Counts 6000 - =121 mm detector 2000 - (E, θw) ddet window Distance/2+20 (mm) θw θlab dreaction 2 |P 4(cos( θcm )| θcm 6He+ 4He at 7.5 MeV – 7Li+ 7Li the 10.15 MeV state in 10 Be N. -

The Skyrme Model Fundamentals Methods Applications
springer.com Physics : Elementary Particles, Quantum Field Theory Makhankov, V.G., Rybakov, Y.P., Sanyuk, V.I. The Skyrme Model Fundamentals Methods Applications The December 1988 issue of the International Journal of Modern Physics A is dedicated to the memory of Tony Hilton Royle Skyrme. It contains an informative account of his life by Dalitz and Aitchison's reconstruction of a talk by Skyrme on the origin of the Skyrme model. From these pages, we learn that Tony Skyrme was born in England in December 1922. He grew up in that country during a period of increasing economic and political turbulence in Europe and elsewhere. In 1943, after Cambridge, he joined the British war effort in making the atomic bomb. He was associated with military projects throughout the war years and began his career as an academic theoretical physicist only in 1946. During 1946-61, he was associated with Cambridge, Birmingham and Harwell and was engaged in wide-ranging investigations in nuclear physics. It was this research which eventually culminated in his studies of nonlinear field theories and his remarkable proposals for the description of the nucleon as a chiral soliton. In his talk, Skyrme described the reasons behind his extraordinary sug• gestions, which Springer when first made must have seemed bizarre. According to him, ideas of this sort go back many Softcover reprint of the decades and occur in the work of Sir William Thomson, who later became Lord Kelvin. Skyrme 1st original 1st ed. 1993, XVIII, edition had heard of Kelvin in his youth. 265 p. Order online at springer.com/booksellers Springer Nature Customer Service Center LLC Printed book 233 Spring Street Softcover New York, NY 10013 Printed book USA Softcover T: +1-800-SPRINGER NATURE ISBN 978-3-642-84672-4 (777-4643) or 212-460-1500 [email protected] $ 109,99 Available Discount group Professional Books (2) Product category Monograph Series Springer Series in Nuclear and Particle Physics Other renditions Softcover ISBN 978-3-642-84671-7 Prices and other details are subject to change without notice. -

The MUSE Experiment and the Proton Radius
Introduction Resolutions to the Puzzle MUSE - MUon Scattering Experiment The MUSE experiment and the Proton Radius Cristina Collicott SPIN 2016 Cristina Collicott SPIN 2016 1/17 Introduction Resolutions to the Puzzle MUSE - MUon Scattering Experiment The Proton Radius Puzzle How big is the proton? Easy question to ask, not so easy to answer! Currently an unanswered problem in physics Cristina Collicott SPIN 2016 2/17 Introduction Resolutions to the Puzzle MUSE - MUon Scattering Experiment The Proton Radius Puzzle What is the proton radius puzzle? The proton charge radius, measured via muonic hydrogen spectroscopy, is 4% smaller than results from hydrogen spectroscopy and elastic electron proton scattering experiments. Cristina Collicott SPIN 2016 3/17 Introduction Resolutions to the Puzzle MUSE - MUon Scattering Experiment The Proton Radius Puzzle - scattering Rosenbluth scattering: 2 2 2 2 dσ dσ GE (Q )+ xGM (Q ) 2 2 2 θ = + 2xGM (Q ) tan dΩ dΩ (point.) 1 + x 2 Sach's form factors (F1,F2 Dirac and Pauli FFs) 2 2 2 GE (Q ) = F1(Q ) − xF2(Q ) 2 2 2 GE (Q ) = F1(Q ) − xF2(Q ) hq 2 x = 2Mc Cristina Collicott SPIN 2016 4/17 Introduction Resolutions to the Puzzle MUSE - MUon Scattering Experiment The Proton Radius Puzzle - scattering Rosenbluth scattering: 2 2 2 2 dσ dσ GE (Q )+ xGM (Q ) 2 2 2 θ = + 2xGM (Q ) tan dΩ dΩ (point.) 1 + x 2 Derivative in the Q2 ! 0 limit 2 2 dGE (Q ) < rE >= −6 2 dQ Q2!0 We expect identical results for hq 2 experiments with ep and µp x = 2Mc scattering.. -
![Arxiv:2103.17101V1 [Physics.Hist-Ph] 29 Mar 2021 1/2 1/2 Are Equal, While That of 2P3/2 Is Higher by About 40 Μev; This Is the fine Structure (FS), Fig](https://docslib.b-cdn.net/cover/7668/arxiv-2103-17101v1-physics-hist-ph-29-mar-2021-1-2-1-2-are-equal-while-that-of-2p3-2-is-higher-by-about-40-ev-this-is-the-ne-structure-fs-fig-217668.webp)
Arxiv:2103.17101V1 [Physics.Hist-Ph] 29 Mar 2021 1/2 1/2 Are Equal, While That of 2P3/2 Is Higher by About 40 Μev; This Is the fine Structure (FS), Fig
April 1, 2021 0:46 WSPC Proceedings - 9in x 6in protonr page 1 1 THE QUEST FOR THE PROTON CHARGE RADIUS ISTVAN´ ANGELI Institute of Experimental Physics, University of Debrecen, Hungary A slight anomaly in optical spectra of the hydrogen atom led Willis E. Lamb to the search for the proton size. As a result, he found the shift of the 2S1/2 level, the first experimental demonstration of quantum electrodynamics (QED). In return, a modern test of QED yielded a new value of the charge radius of the proton. This sounds like Baron M¨unchausen's tale: to pull oneself out from the marsh by seizing his own hair. An independent method was necessary. Muonic hydrogen spectroscopy came to the aid. However, the high-precision result significantly differed from the previous { electronic { values: this is (was?) the proton radius puzzle (2010-2020?). This puzzle produced a decade-long activity both in experimental work and in theory. Even if the puzzle seems to be solved, the precise determination of the proton charge radius requires further efforts in the future. 1. The Dirac equation; anomalies in hydrogen spectra (1928{1938) In 1928, P.A.M. Dirac published his relativistic wave equation implying two important consequences: (1) The electron has an intrinsic magnetic dipole moment µe = 1 × µB (µB: Bohr magneton) in agreement with the experiment (1925: George Uhlenbeck, Samuel Goudsmit). (2) If in the hydrogen atom the electron moves in the field of a Coulomb potential V (r) ∼ 1=r, then its energy E(n; j) is determined by the principal quantum number n and the total angular momentum quan- tum number j, but not by the orbital angular momentum l and spin s, separately. -

The Proton Radius Puzzle Μ
The proton radius puzzle µ A. Antognini Paul Scherrer Institute ETH, Zurich Switzerland CREMA collaboration Laser spectroscopy of muonic atoms µ 2P 2S-2P Laser excitation Energy 2S 1S μ • 2S-2P p From 2S-2P • 2S-2P μd ꔄ charge radii • 2S-2P μ3He, μ4He Aldo Antognini Rencontres de Moriond 19.03.2017 2 Three ways to the proton radius p p e- H 2 e- µ- e--p scattering H spectroscopy µp spectroscopy 6.7 σ CODATA-2010 µp 2013 scatt. JLab µp 2010 scatt. Mainz H spectroscopy 0.82 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 Proton charge radius [fm] Pohl et al., Nature 466, 213 (2010) Antognini et al., Science 339, 417 (2013) Pohl et al., Science 353, 669 (2016) Aldo Antognini Rencontres de Moriond 19.03.2017 3 Extracting the proton radius from 훍p Measure 2S-2P splitting (20 ppm) 8.4 meV 2P F=2 and compare with theory 3/2 F=1 2P1/2 F=1 → proton radius F=0 ∆Eth . − . r2 . 2P −2S = 206 0336(15) 5 2275(10) p +00332(20) [meV] 206 meV 50 THz 6 µm fin. size: 3.8 meV F=1 2π(Zα) 2 2 2S ∆Esize = r |Ψnl(0)| 1/2 m ≈ 200m 3 p 23 meV µ e 4 2(Zα) 3 2 F=0 3 m r δl = 3n r p 0 Aldo Antognini Rencontres de Moriond 19.03.2017 4 Principle of the µp 2S-2P experiment Produce many µ− at keV energy − Form µp by stopping µ in 1 mbar H2 gas Fire laser to induce the 2S-2P transition Measure the 2 keV X-rays from 2P-1S decay µp formation Laser excitation Plot number of X-rays vs laser frequency ] n~14 2 P -4 7 Laser 6 1 % 99 % 2 S 5 2 P 4 2 S 3 2 keV 2 keV γ γ 2 delayed / prompt events [10 1 0 1 S 49.75 49.8 49.85 49.9 49.95 1 S laser frequency [THz] Aldo Antognini Rencontres de Moriond 19.03.2017 5 The setup at the Paul Scherrer Institute Aldo Antognini Rencontres de Moriond 19.03.2017 6 The first 훍p resonance (2010) Discrepancy: 5.0 σ ↔ 75 GHz ↔ δν/ν =1.5 × 10−3 ] -4 7 CODATA-06 our value 6 e-p scattering H2O 5 calib. -

The Proton Radius Puzzle-Why We All Should Care
SNSN-323-63 September 27, 2018 The Proton Radius Puzzle- Why We All Should Care Gerald A. Miller1 Physics Department, University of Washington, Seattle, Washington 98195-1560, USA The status of the proton radius puzzle (as of the date of the Confer- ence) is reviewed. The most likely potential theoretical and experimental explanations are discussed. Either the electronic hydrogen experiments were not sufficiently accurate to measure the proton radius, the two- photon exchange effect was not properly accounted for, or there is some kind of new physics. I expect that upcoming experiments will resolve this issue within the next year or so. PRESENTED AT Conference on the Intersections between particle and nuclear physics, arXiv:1809.09635v1 [physics.atom-ph] 25 Sep 2018 Indian Wells, USA, May 29{ June 3, 2018 1This work was supported by the U. S. Department of Energy Office of Science, Office of Nuclear Physics under Award Number DE-FG02-97ER-41014,. This title is chosen because understanding of the proton radius puzzle requires knowledge of atomic, nuclear and particle physics. The puzzle began with the pub- lication of the results of the 2010 muon-hydrogen experiment in 2010 [1] and its confirmation [2]. The proton radius (r2 = 1=6G0 (Q2 = 0) was measured to be p − E rp = 0:84184(67) fm, which contrasted with the value obtained from electron spec- troscopy rp = 0:8768(69) fm. This difference of about 4% has become known as the proton radius puzzle [3]. We use the technical terms: the radius 0.87 fm is denoted as large, and the one of 0.84 fm as small. -

The Proton Radius Puzzle and the Electro-Strong Interaction
The Proton Radius Puzzle and the Electro-Strong Interaction The resolution of the Proton Radius Puzzle is the diffraction pattern, giving another wavelength in case of muonic hydrogen oscillation for the proton than it is in case of normal hydrogen because of the different mass rate. Taking into account the Planck Distribution Law of the electromagnetic oscillators, we can explain the electron/proton mass rate and the Weak and Strong Interactions. Lattice QCD gives the same results as the diffraction patterns of the electromagnetic oscillators, explaining the color confinement and the asymptotic freedom of the Strong Interactions. Contents Preface ................................................................................................................................... 2 The Proton Radius Puzzle ......................................................................................................... 2 Asymmetry in the interference occurrences of oscillators ............................................................ 2 Spontaneously broken symmetry in the Planck distribution law .................................................... 4 The structure of the proton ...................................................................................................... 6 The weak interaction ............................................................................................................... 6 The Strong Interaction - QCD .................................................................................................... 7 Confinement -
![Arxiv:1909.08108V3 [Hep-Ph] 26 Sep 2019 Value Obtained from Muonic Hydrogen Is Re = 0.84087(39) Fm [8], While the Most Recent CODATA P Value Is Re = 0.8751(61) Fm [9]](https://docslib.b-cdn.net/cover/9074/arxiv-1909-08108v3-hep-ph-26-sep-2019-value-obtained-from-muonic-hydrogen-is-re-0-84087-39-fm-8-while-the-most-recent-codata-p-value-is-re-0-8751-61-fm-9-829074.webp)
Arxiv:1909.08108V3 [Hep-Ph] 26 Sep 2019 Value Obtained from Muonic Hydrogen Is Re = 0.84087(39) Fm [8], While the Most Recent CODATA P Value Is Re = 0.8751(61) Fm [9]
The Proton Radius Puzzle Gil Paz Department of Physics and Astronomy, Wayne State University, Detroit, Michigan 48201, USA Abstract: In 2010 the proton charge radius was extracted for the first time from muonic hydrogen, a bound state of a muon and a proton. The value obtained was five standard deviations away from the regular hydrogen extraction. Taken at face value, this might be an indication of a new force in nature coupling to muons, but not to electrons. It also forces us to reexamine our understanding of the structure of the proton. Here I describe an ongoing theoretical research effort that seeks to address this \proton radius puzzle". In particular, I will present the development of new effective field theoretical tools that seek to directly connect muonic hydrogen and muon-proton scattering. Talk presented at the 2019 Meeting of the Division of Particles and Fields of the American Physical Society (DPF2019), July 29{August 2, 2019, Northeastern University, Boston, C1907293. 1 Introduction How big is the proton? To answer such a question one needs to define how the proton size is measured. For example, one can use an electromagnetic probe to determine the proton's size. A \one photon" electromagnetic interaction with an on-shell proton can be described by two form 2 factors: F1 and F2. These form factors are functions of q , the square of the four-momentum transfer. Two different linear combinations of F1 and F2 define the \electric" form factor: GE = 2 2 F1 + q F2=4M , where M is the proton mass, and the \magnetic" form factor: GM = F1 + F2. -

Topology in Physics-A Perspective
SU-4228-533 March 1993 TOPOLOGY IN PHYSICS - A PERSPECTIVE A.P. Balachandran Department of Physics, Syracuse University, Syracuse, NY 13244-1130 ABSTRACT This article, written in honour of Fritz Rohrlich, briefly surveys the role of topology in physics. arXiv:hep-th/9303177v1 31 Mar 1993 When I joined Syracuse University as a junior faculty member in September, 1964, Fritz Rohrlich was already there as a senior theoretician in the quantum field theory group. He was a very well-known physicist by that time, having made fundamental contributions to quantum field theory, and written his splendid book with Jauch. My generation of physicists grew up with Jauch and Rohrlich, and I was also familiar with Fritz’s research. It was therefore with a certain awe and a great deal of respect that I first made his acquaintance. Many years have passed since this first encounter. Our interests too have gradually evolved and changed in this intervening time. Starting from the late seventies or there- abouts, particle physicists have witnessed an increasing intrusion of topological ideas into their discipline. Our group at Syracuse has responded to this development by getting involved in soliton and monopole physics and in investigations on the role of topology in quantum physics. Meanwhile, especially during the last decade, there has been a per- ceptible shift in the direction of Fritz’s research to foundations and history of quantum physics. Later I will argue that topology affects the nature of wave functions (or more accurately of wave functions in the domains of observables) and has a profound meaning for the fundamentals of quantum theory. -
Quark•Gluon Plasma
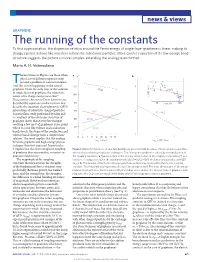
news & views GRAPHENE The running of the constants To first approximation, the dispersion relation around the Fermi energy of single-layer graphene is linear, making its charge carriers behave like massless relativistic subatomic particles. More careful inspection of its low-energy band structure suggests the picture is more complex, extending the analogy even further. Maria A. H. Vozmediano he best times in Physics are those when a α–1 b physicists of different expertise meet around a problem of common interest. 3 T ) And this is now happening in the case of 120 3 –1 α–1 2 graphene. From the early days of the isolation QED ms of single sheets of graphene, the relativistic 6 100 (10 1 F nature of its charge carriers was clear . v These carriers, known as Dirac fermions, are 1 described by equations similar to those that 80 10 E (meV) 100 describe the quantum electrodynamic (QED) interactions of relativistic charged particles. 60 2 U(1) ѵ ~ α–1 A meticulous study performed by Elias and F G 2 co-workers of the electronic structure of 40 SU(2) graphene shows that at very low energies reaching a few meV of graphene’s Dirac point, 20 where its cone-like valence and conduction SU(3) bands touch, the shape of the conduction and valence bands diverge from a simple linear 1 0 2 4 6 8 10 12 14 16 1 2 relation. The result implies that the analogy log E (Gev) log E (10–12 Gev) between graphene and high-energy physics 10 10 is deeper than first expected. -

Bertlmann – a Nonlocal Quantum Engineer
a nonlocal quantum engineer contemplate but don’t forget to calculate ! Reinhold A. Bertlmann Faculty of Physics, University of Vienna talk at symposium “ shut up and contemplate ! ” University of Vienna, March 3rd, 2017 engineer’s career at Harwell John Stewart Bell graduated at Queen’s University Belfast in experimental physics 1948 in mathematical physics 1949 high interest in quantum mechanics showing his dissatisfaction ! position at AERE Harwell from1949 on joined accelerator group of Walkinshaw (Malvern) William Walkinshaw: John on his new “Ariel” “ a young man of high caliber who showed his at Harwell 1952 independence … his mathematical talent was superb and elegant.” papers on electron and proton linear accelerators e.g. “basic algebra on strong focussing systems” “linear accelerator phase oscillations” “phase debunching by focussing foils in proton linear accelerator” Reinhold A. Bertlmann 2 turn to particle physics & Bohm’s qm in mid1950s Bell turned to nuclear and particle physics “time reversal in field theory …”, CPT theorem, PhD thesis fundamental ! “anomalous magnetic moments of nucleons”, collab. Tony Skyrme at that time paper of David Bohm appeared 1952 “interpretation of quantum theory in terms of hidden variable” Mary: for John a “revelation” “everything has definite properties” I remember John saying Bell’s talk about Bohm’s theory in TH division fierce debates with audience especially with Franz Mandl 1954 John married Mary Ross “mathematical engineer” accelerator physicist Reinhold A. Bertlmann 3 engineer’s job -

Aspects of the Many-Body Problem in Nuclear Physics
Aspects of the Many-Body Problem in Nuclear Physics DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Alexander Signatur Dyhdalo, MSc, BSc Graduate Program in Physics The Ohio State University 2018 Dissertation Committee: Prof. Richard Furnstahl, Advisor Prof. Robert Perry Prof. Michael Lisa Prof. Junko Shigemitsu c Copyright by Alexander Signatur Dyhdalo 2018 Abstract Low-energy nuclear physics has seen a renaissance of activity recently with the advent of the nuclear effective field theory (EFT) approach, the power of renormalization group techniques, and advances in the computational cost-effectiveness and sophistication of quan- tum many-body methods. Nevertheless challenges remain in part from ambiguities of the nuclear Hamiltonian via regulator artifacts and the scaling of the many-body methods to heavier systems. Regulator artifacts arise due to a renormalization inconsistency in the nuclear EFT as currently formulated in Weinberg power counting, though their ultimate impact on nuclear observables is unknown at present. This results in a residual cutoff dependence in the EFT to all orders. We undertake an examination of these regulator artifacts using perturba- tive energy calculations of uniform matter as a testbed. Our methodology has found that the choice of regulator determines the shape of the energy phase space and that different regulators weight distinct phase space regions differently. Concerning many-body calculations, at present only phenomenological energy density functionals are able to solve for the full table of nuclides. However these functionals are currently unconnected to QCD and have no existing method for systematic improvement.