Following Carbon's Evolutionary Path: From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
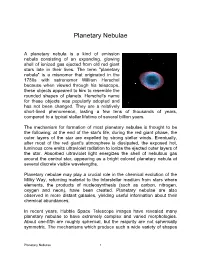
Planetary Nebulae
Planetary Nebulae A planetary nebula is a kind of emission nebula consisting of an expanding, glowing shell of ionized gas ejected from old red giant stars late in their lives. The term "planetary nebula" is a misnomer that originated in the 1780s with astronomer William Herschel because when viewed through his telescope, these objects appeared to him to resemble the rounded shapes of planets. Herschel's name for these objects was popularly adopted and has not been changed. They are a relatively short-lived phenomenon, lasting a few tens of thousands of years, compared to a typical stellar lifetime of several billion years. The mechanism for formation of most planetary nebulae is thought to be the following: at the end of the star's life, during the red giant phase, the outer layers of the star are expelled by strong stellar winds. Eventually, after most of the red giant's atmosphere is dissipated, the exposed hot, luminous core emits ultraviolet radiation to ionize the ejected outer layers of the star. Absorbed ultraviolet light energizes the shell of nebulous gas around the central star, appearing as a bright colored planetary nebula at several discrete visible wavelengths. Planetary nebulae may play a crucial role in the chemical evolution of the Milky Way, returning material to the interstellar medium from stars where elements, the products of nucleosynthesis (such as carbon, nitrogen, oxygen and neon), have been created. Planetary nebulae are also observed in more distant galaxies, yielding useful information about their chemical abundances. In recent years, Hubble Space Telescope images have revealed many planetary nebulae to have extremely complex and varied morphologies. -

GALEX Helix Nebula Poster
National Aeronautics and Space Administration The Helix Nebula: What is it? The object shown on the front of this poster is the This “Mountains Helix Nebula. Although this object and others like it are of Creation” image called planetary nebulae (pronounced NEB-u-lee), they was captured by really have nothing to do with planets. They got their name the Spitzer Space ZKHQDVWURQRPHUV¿rst saw them through early telescopes, Telescope in infrared because they looked similar to planets with rings around light. It reveals them, like Saturn. billowing mountains of dust ablaze with A planetary nebula is the ¿res of active star really a shell of glowing gas formation. GALEX and plasma from a star at the can see the new stars end of its life. The star has forming, because they glow brightly in ultraviolet (UV) light. blown off much of its material However, the surrounding dust and gas clouds are cooler and and what is left is a very not so visible to GALEX. compact object called a white dwarf. For a while, the white dwarf is still hot and bright A Tug of War enough to make the material Planetary nebula JnEr1, A star is an amazing from the former star glow, as seen by GALEX. ! Ow! If not for balancing act between two ! * * gravity, my head and that is what we see as a * * would explode! beautiful nebula. Over 10,000 huge forces. On the one * years or so, the gas will drift away and the white dwarf will hand, the crushing force of the star’s own gravity Gravity Heat cool so much that we can no longer see the nebula. -

Poster Abstracts
Aimée Hall • Institute of Astronomy, Cambridge, UK 1 Neptunes in the Noise: Improved Precision in Exoplanet Transit Detection SuperWASP is an established, highly successful ground-based survey that has already discovered over 80 exoplanets around bright stars. It is only with wide-field surveys such as this that we can find planets around the brightest stars, which are best suited for advancing our knowledge of exoplanetary atmospheres. However, complex instrumental systematics have so far limited SuperWASP to primarily finding hot Jupiters around stars fainter than 10th magnitude. By quantifying and accounting for these systematics up front, rather than in the post- processing stage, the photometric noise can be significantly reduced. In this paper, we present our methods and discuss preliminary results from our re-analysis. We show that the improved processing will enable us to find smaller planets around even brighter stars than was previously possible in the SuperWASP data. Such planets could prove invaluable to the community as they would potentially become ideal targets for the studies of exoplanet atmospheres. Alan Jackson • Arizona State University, USA 2 Stop Hitting Yourself: Did Most Terrestrial Impactors Originate from the Terrestrial Planets? Although the asteroid belt is the main source of impactors in the inner solar system today, it contains only 0.0006 Earth mass, or 0.05 Lunar mass. While the asteroid belt would have been much more massive when it formed, it is unlikely to have had greater than 0.5 Lunar mass since the formation of Jupiter and the dissipation of the solar nebula. By comparison, giant impacts onto the terrestrial planets typically release debris equal to several per cent of the planet’s mass. -

Two Rings but No Fellowship: Lotr 1 and Its Relation to Planetary Nebulae
Mon. Not. R. Astron. Soc. 000, 1–16 (2013) Printed 17 October 2018 (MN LATEX style file v2.2) Two rings but no fellowship: LoTr 1 and its relation to planetary nebulae possessing barium central stars. A.A. Tyndall1,2⋆, D. Jones2, H.M.J. Boffin2, B. Miszalski3,4, F. Faedi5, M. Lloyd1, J.A. L´opez6, S. Martell7, D. Pollacco5, and M. Santander-Garc´ıa8 1Jodrell Bank Centre for Astrophysics, School of Physics and Astronomy, University of Manchester, M13 9PL, UK 2European Southern Observatory, Alonso de C´ordova 3107, Casilla 19001, Santiago, Chile 3South African Astronomical Observatory, PO Box 9, Observatory 7935, South Africa 4Southern African Large Telescope. PO Box 9, Observatory 7935, South Africa 5Department of Physics, University of Warwick, CV4 7AL, UK 6Instituto de Astronom´ıa, Universidad Nacional Aut´onoma de M´exico, Ensenada, Baja California, C.P. 22800, Mexico 7Australian Astronomical Observatory, North Ryde, 2109 NSW, Australia 8Observatorio Astron´omico National, Madrid, and Centro de Astrobiolog´ıa, CSIC-INTA, Spain Accepted xxxx xxxxxxxx xx. Received xxxx xxxxxxxx xx; in original form xxxx xxxxxxxx xx ABSTRACT LoTr 1 is a planetary nebula thought to contain an intermediate-period binary central star system ( that is, a system with an orbital period, P, between 100 and, say, 1500 days). The system shows the signature of a K-type, rapidly rotating giant, and most likely constitutes an accretion-induced post-mass transfer system similar to other PNe such as LoTr 5, WeBo 1 and A70. Such systems represent rare opportunities to further the investigation into the formation of barium stars and intermediate period post-AGB systems – a formation process still far from being understood. -

Observatories
NATIONAL OPTICAL ASTRONOMY OBSERVATORIES NATIONAL OPTICAL ASTRONOMY OBSERVATORIES FY 1997 PROVISIONAL PROGRAM PLAN September 19,1996 TABLE OF CONTENTS I. INTRODUCTION AND OVERVIEW 1 II. THE DEVELOPMENT PROGRAM: MILESTONES PAST AND FUTURE 3 IE. NIGHTTIME PROGRAM 6 A. Major Projects 6 1. SOAR ".""".6 2. WIYN 8 B. Joint Nighttime Instrumentation Program 9 1. Overview 9 2. Description of Individual Major Projects 10 3. Detectors 13 4. NOAO Explorations of Technology (NExT) Program 14 C. USGP 15 1. Overview 15 2. US Gemini Instrument Program 16 D. Telescope Operations and User Support 17 1. Changes in User Services 17 2. Telescope Upgrades 18 a. CTIO 18 b. KPNO 21 3. Instrumentation Improvements 24 a. CTIO 24 b. KPNO 26 4. Smaller Telescopes 29 IV. NATIONAL SOLAR OBSERVATORY 29 A. Major Projects 29 1. Global Oscillation Network Group (GONG) 30 2. RISE/PSPT Program 33 3. SOLIS 34 4. Study of CLEAR 35 B. Instrumentation 36 1. General 36 2. Sacramento Peak 36 3. NSO/KittPeak 38 a. Infrared Program 38 b. Telescope Improvement 40 C. Telescope Operations and User Support 41 1. Sacramento Peak 41 2. NSO/KittPeak 42 V. THE SCIENTIFIC STAFF 42 VI. EDUCATIONAL OUTREACH 45 A. Educational Program 45 1. Direct Classroom Involvement 46 2. Development of Instructional Materials 46 3. WWW Distribution of Science Resources 47 4. Outreach Advisory Board 47 5. Undergraduate Education 47 6. Graduate Education 47 7. Educational Partnership Programs 47 B. Public Information 48 1. Press Releases and Other Interactions With the Media 48 2. Images 48 3 Visitor Center 48 C. -

A Basic Requirement for Studying the Heavens Is Determining Where In
Abasic requirement for studying the heavens is determining where in the sky things are. To specify sky positions, astronomers have developed several coordinate systems. Each uses a coordinate grid projected on to the celestial sphere, in analogy to the geographic coordinate system used on the surface of the Earth. The coordinate systems differ only in their choice of the fundamental plane, which divides the sky into two equal hemispheres along a great circle (the fundamental plane of the geographic system is the Earth's equator) . Each coordinate system is named for its choice of fundamental plane. The equatorial coordinate system is probably the most widely used celestial coordinate system. It is also the one most closely related to the geographic coordinate system, because they use the same fun damental plane and the same poles. The projection of the Earth's equator onto the celestial sphere is called the celestial equator. Similarly, projecting the geographic poles on to the celest ial sphere defines the north and south celestial poles. However, there is an important difference between the equatorial and geographic coordinate systems: the geographic system is fixed to the Earth; it rotates as the Earth does . The equatorial system is fixed to the stars, so it appears to rotate across the sky with the stars, but of course it's really the Earth rotating under the fixed sky. The latitudinal (latitude-like) angle of the equatorial system is called declination (Dec for short) . It measures the angle of an object above or below the celestial equator. The longitud inal angle is called the right ascension (RA for short). -

Astronomy 2008 Index
Astronomy Magazine Article Title Index 10 rising stars of astronomy, 8:60–8:63 1.5 million galaxies revealed, 3:41–3:43 185 million years before the dinosaurs’ demise, did an asteroid nearly end life on Earth?, 4:34–4:39 A Aligned aurorae, 8:27 All about the Veil Nebula, 6:56–6:61 Amateur astronomy’s greatest generation, 8:68–8:71 Amateurs see fireballs from U.S. satellite kill, 7:24 Another Earth, 6:13 Another super-Earth discovered, 9:21 Antares gang, The, 7:18 Antimatter traced, 5:23 Are big-planet systems uncommon?, 10:23 Are super-sized Earths the new frontier?, 11:26–11:31 Are these space rocks from Mercury?, 11:32–11:37 Are we done yet?, 4:14 Are we looking for life in the right places?, 7:28–7:33 Ask the aliens, 3:12 Asteroid sleuths find the dino killer, 1:20 Astro-humiliation, 10:14 Astroimaging over ancient Greece, 12:64–12:69 Astronaut rescue rocket revs up, 11:22 Astronomers spy a giant particle accelerator in the sky, 5:21 Astronomers unearth a star’s death secrets, 10:18 Astronomers witness alien star flip-out, 6:27 Astronomy magazine’s first 35 years, 8:supplement Astronomy’s guide to Go-to telescopes, 10:supplement Auroral storm trigger confirmed, 11:18 B Backstage at Astronomy, 8:76–8:82 Basking in the Sun, 5:16 Biggest planet’s 5 deepest mysteries, The, 1:38–1:43 Binary pulsar test affirms relativity, 10:21 Binocular Telescope snaps first image, 6:21 Black hole sets a record, 2:20 Black holes wind up galaxy arms, 9:19 Brightest starburst galaxy discovered, 12:23 C Calling all space probes, 10:64–10:65 Calling on Cassiopeia, 11:76 Canada to launch new asteroid hunter, 11:19 Canada’s handy robot, 1:24 Cannibal next door, The, 3:38 Capture images of our local star, 4:66–4:67 Cassini confirms Titan lakes, 12:27 Cassini scopes Saturn’s two-toned moon, 1:25 Cassini “tastes” Enceladus’ plumes, 7:26 Cepheus’ fall delights, 10:85 Choose the dome that’s right for you, 5:70–5:71 Clearing the air about seeing vs. -

September 2020 BRAS Newsletter
A Neowise Comet 2020, photo by Ralf Rohner of Skypointer Photography Monthly Meeting September 14th at 7:00 PM, via Jitsi (Monthly meetings are on 2nd Mondays at Highland Road Park Observatory, temporarily during quarantine at meet.jit.si/BRASMeets). GUEST SPEAKER: NASA Michoud Assembly Facility Director, Robert Champion What's In This Issue? President’s Message Secretary's Summary Business Meeting Minutes Outreach Report Asteroid and Comet News Light Pollution Committee Report Globe at Night Member’s Corner –My Quest For A Dark Place, by Chris Carlton Astro-Photos by BRAS Members Messages from the HRPO REMOTE DISCUSSION Solar Viewing Plus Night Mercurian Elongation Spooky Sensation Great Martian Opposition Observing Notes: Aquila – The Eagle Like this newsletter? See PAST ISSUES online back to 2009 Visit us on Facebook – Baton Rouge Astronomical Society Baton Rouge Astronomical Society Newsletter, Night Visions Page 2 of 27 September 2020 President’s Message Welcome to September. You may have noticed that this newsletter is showing up a little bit later than usual, and it’s for good reason: release of the newsletter will now happen after the monthly business meeting so that we can have a chance to keep everybody up to date on the latest information. Sometimes, this will mean the newsletter shows up a couple of days late. But, the upshot is that you’ll now be able to see what we discussed at the recent business meeting and have time to digest it before our general meeting in case you want to give some feedback. Now that we’re on the new format, business meetings (and the oft neglected Light Pollution Committee Meeting), are going to start being open to all members of the club again by simply joining up in the respective chat rooms the Wednesday before the first Monday of the month—which I encourage people to do, especially if you have some ideas you want to see the club put into action. -

SPACE NEWS Previous Page | Contents | Zoom in | Zoom out | Front Cover | Search Issue | Next Page BEF Mags INTERNATIONAL
Contents | Zoom in | Zoom out For navigation instructions please click here Search Issue | Next Page SPACEAPRIL 19, 2010 NEWSAN IMAGINOVA CORP. NEWSPAPER INTERNATIONAL www.spacenews.com VOLUME 21 ISSUE 16 $4.95 ($7.50 Non-U.S.) PROFILE/22> GARY President’s Revised NASA Plan PAYTON Makes Room for Reworked Orion DEPUTY UNDERSECRETARY FOR SPACE PROGRAMS U.S. AIR FORCE AMY KLAMPER, COLORADO SPRINGS, Colo. .S. President Barack Obama’s revised space plan keeps Lockheed Martin working on a Ulifeboat version of a NASA crew capsule pre- INSIDE THIS ISSUE viously slated for cancellation, potentially positioning the craft to fly astronauts to the interna- tional space station and possibly beyond Earth orbit SATELLITE COMMUNICATIONS on technology demonstration jaunts the president envisions happening in the early 2020s. Firms Complain about Intelsat Practices Between pledging to choose a heavy-lift rocket Four companies that purchase satellite capacity from Intelsat are accusing the large fleet design by 2015 and directing NASA and Denver- operator of anti-competitive practices. See story, page 5 based Lockheed Martin Space Systems to produce a stripped-down version of the Orion crew capsule that would launch unmanned to the space station by Report Spotlights Closed Markets around 2013 to carry astronauts home in an emer- The office of the U.S. Trade Representative has singled out China, India and Mexico for not meet- gency, the White House hopes to address some of the ing commitments to open their domestic satellite services markets. See story, page 13 chief complaints about the plan it unveiled in Feb- ruary to abandon Orion along with the rest of NASA’s Moon-bound Constellation program. -

A Guide to Star Names, Pronunciations, and Related Constellations
Astronomy Club of Asheville 3 Feb. 2020 version Page 1 of 8 A Guide to Star Names, Pronunciations, and Related Constellations Proper Star Names: There are over 300 proper star names that are used and accepted worldwide today -- and approved by the International Astronomical Union. Most of them come from the ancient Arabic cultures, but there are many star names in common use from the Greek, the Roman, European, and other cultures as well. On the pages below you will find an alphabetical list of many, but not all, of the proper star names, their pronunciations, and related constellations. Find a list of star names by constellation at this web link. Find another list of common star names at this web link. Star Catalogs that are Currently Used by Astronomers 1. Bayer Catalog: This first widely recognized star catalog was published by German astronomer Johann Bayer in 1603. This list of naked-eye stars indexes the stars in each constellation, using the letters of the Greek alphabet, followed by the genitive form of its parent constellation's Latin name, e.g., alpha Orionis. Typically, the brightest star in each constellation received the first Greek letter (alpha), the second brightest star the second letter (beta), and on-and-on. But you will often notice that the Bayer sequencing of the bright stars in the constellations is not true to what we see and know today about their apparent brightness. Original errors in the brightness estimates, along with the brightness variability of the stars (like Betelgeuse in Orion), have caused the Bayer sequencing not to match the stellar brightness that we observe today. -

IPHAS Extinction Distances to Planetary Nebulae
A&A 525, A58 (2011) Astronomy DOI: 10.1051/0004-6361/201014464 & c ESO 2010 Astrophysics IPHAS extinction distances to planetary nebulae C. Giammanco1,12,S.E.Sale2,R.L.M.Corradi1,12,M.J.Barlow3, K. Viironen1,13,14,L.Sabin4, M. Santander-García5,1,12,D.J.Frew6,R.Greimel7, B. Miszalski10, S. Phillipps8, A. A. Zijlstra9, A. Mampaso1,12,J.E.Drew2,10,Q.A.Parker6,11, and R. Napiwotzki10 1 Instituto de Astrofísica de Canarias (IAC), C/ vía Láctea s/n, 38200 La Laguna, Spain e-mail: [email protected] 2 Astrophysics Group, Imperial College London, Blackett Laboratory, Prince Consort Road, London SW7 2AZ, UK 3 Department of Physics and Astronomy, University College London, Gower Street, London WC1E 6BT, UK 4 Instituto de Astronomía, Universidad Nacional Autónoma de México, Apdo. Postal 877, 22800 Ensenada, B.C., Mexico 5 Isaac Newton Group of Telescopes, Ap. de Correos 321, 38700 Sta. Cruz de la Palma, Spain 6 Department of Physics, Macquarie University, NSW 2109, Australia 7 Institut für Physik, Karl-Franzens Universität Graz, Universitätsplatz 5, 8010 Graz, Austria 8 Astrophysics Group, Department of Physics, Bristol University, Tyndall Avenue, Bristol BS8 1TL, UK 9 Jodrell Bank Centre for Astrophysics, School of Physics and Astronomy, University of Manchester, Oxford Road, M13 9PL Manchester, UK 10 Centre for Astrophysics Research, STRI, University of Hertfordshire, College Lane Campus, Hatfield AL10 9AB, UK 11 Anglo-Australian Observatory, PO Box 296, Epping, NSW 1710, Australia 12 Departamento de Astrofísica, Universidad de La Laguna, 38205 La Laguna, Tenerife, Spain 13 Centro Astronómico Hispano Alemán, Calar Alto, C/Jesús Durbán Remón 2-2, 04004 Almeria, Spain 14 Centro de Estudios de Física del Cosmos de Aragón (CEFCA), C/General Pizarro 1-1, 44001 Teruel, Spain Received 19 March 2010 / Accepted 3 July 2010 ABSTRACT Aims. -

The Double Helix Nebula: a Magnetic Torsional Wave Propagating out of the Galactic Centre
The Double Helix Nebula: a magnetic torsional wave propagating out of the Galactic centre Mark Morris1, Keven Uchida2, and Tuan Do1 1Department of Physics and Astronomy, University of California, Los Angeles, Los Angeles, CA 90095-1547, USA 2Center for Radiophysics and Space Research, Cornell University, Space Sciences Building, Ithaca, NY 14853-6801 Radioastronomical studies have indicated that the magnetic field in the central few hundred parsecs of our Milky Way Galaxy has a dipolar geometry and a strength substantially larger than elsewhere in the Galaxy, with estimates ranging up to a milligauss1-6. A strong, large-scale magnetic field can affect the Galactic orbits of molecular clouds by exerting a drag on them, it can inhibit star formation, and it can guide a wind of cosmic rays away from the central region, so a characterization of the magnetic field at the Galactic center is important for understanding much of the activity there. Here, we report Spitzer Space Telescope observations of an unprecedented infrared nebula having the morphology of an intertwined double helix. This feature is located about 100 pc from the Galaxy’s dynamical centre toward positive Galactic latitude, and its axis is oriented perpendicular to the Galactic plane. The observed segment is about 25 pc in length, and contains about 1.25 full turns of each of the two continuous, helically wound strands. We interpret this feature as a torsional Alfvén wave propagating vertically away from the Galactic disk, driven by rotation of the magnetized circumnuclear gas disk. As such, it offers a new morphological probe of the Galactic center magnetic field.