Laboratory Glassware
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atomic Layer Deposition on Dispersed Materials in Liquid Phase by Stoichiometrically Limited Injections
COMMUNICATION www.advmat.de Atomic Layer Deposition on Dispersed Materials in Liquid Phase by Stoichiometrically Limited Injections Benjamin P. Le Monnier, Frederick Wells, Farzaneh Talebkeikhah, and Jeremy S. Luterbacher* deposition.[2] Since then, processes for Atomic layer deposition (ALD) is a well-established vapor-phase technique depositing a range of materials including for depositing thin films with high conformality and atomically precise oxides, nitrides, hybrids and even metals [3–5] control over thickness. Its industrial development has been largely confined have been developed. This wide to wafers and low-surface-area materials because deposition on high-surface- variety of materials combined with its atomic-level precision has made ALD area materials and powders remains extremely challenging. Challenges with a formidable tool for the fabrication of such materials include long deposition times, extensive purging cycles, and nanostructured materials such as transis- requirements for large excesses of precursors and expensive low-pressure tors, solar cells and fuel cells.[6] equipment. Here, a simple solution-phase deposition process based on More recently, processes have been subsequent injections of stoichiometric quantities of precursor is performed developed to apply ALD to high-surface- area materials (>10 m2 g−1), including using common laboratory synthesis equipment. Precisely measured precursor powders for various applications, from stoichiometries avoid any unwanted reactions in solution and ensure layer-by- passivation of photoactive material to cata- layer growth with the same precision as gas-phase ALD, without any excess lyst preparation.[7,8] With such materials, precursor or purging required. Identical coating qualities are achieved when long exposure time (minutes vs millisec- onds for wafers), both for purge and reac- comparing this technique to Al2O3 deposition by fluidized-bed reactor ALD tion cycles, must be coupled with effec- (FBR-ALD). -

Solvent Extraction Instruments Brochure
ROT-X-TRACT SERİES Solvent Extraction Instruments PRODUCT BROCHURE ELEMENTEL ANALİTİK ve BİO TEKNOLOJİK SİSTEMLER | Folkart Towers - Adalet Mah. Manas Blv. No: 39/3408 Bayraklı/IZMIR, TURKEY, PHONE-FAX: +90 232 472 17 11 • [email protected] • www.elementel.com 2 Content www.elementel.com CONTENT ORGANOMATION SOLVENT EXTRACTORS OVERVIEW ..................................................................................... 3 ROT-X-TRACT-S SERIES ROTARY SOLID-LIQUID SOXHLET EXTRACTORS ........................................................ 5 ROT-X-TRACT-LC SERIES CORNING ACCELERATED ONE-STEP LIQUID-LIQUID EXTRACTORS ....................... 7 ROT-X-TRACT SOLVENT EXTRACTION INSTRUMENTS ACCESSORIES AND REPLACEMENT PARTS ............ 9 Organomation Solvent Extraction Instruments Overview 3 www.elementel.com Organomation Solvent Extractors Overview You are in the lab and need to run multiple extractions at once. First you set the Soxhlet extractors up on individual heating mantles or baths. Next, tubing is individually attached to each of the condensers. There are dozens of tubes to deal with, and multiple heating mantles to monitor. The problem with this arrangement is that in the end, you have spent more time assembling and monitoring your apparatuses than performing the actual chemistry. To conserve valuable bench space, all samples are arranged in a circle and the instrument rotates allowing each sample to be accessed from the front. Individual condensers are connected to the centrally located water manifold through quick disconnect fittings. -

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Method 23 Determination of Polychlorinated Dibenzo-P-Dioxins
Method 23 8/3/2017 While we have taken steps to ensure the accuracy of this Internet version of the document, it is not the official version. To see a complete version including any recent edits, visit: https://www.ecfr.gov/cgi-bin/ECFR?page=browse and search under Title 40, Protection of Environment. METHOD 23—DETERMINATION OF POLYCHLORINATED DIBENZO-P-DIOXINS AND POLYCHLORINATED DIBENZOFURANS FROM STATIONARY SOURCES 1. Applicability and Principle 1.1 Applicability. This method is applicable to the determination of polychlorinated dibenzo-p- dioxins (PCDD's) and polychlorinated dibenzofurans (PCDF's) from stationary sources. 1.2 Principle. A sample is withdrawn from the gas stream isokinetically and collected in the sample probe, on a glass fiber filter, and on a packed column of adsorbent material. The sample cannot be separated into a particle vapor fraction. The PCDD's and PCDF's are extracted from the sample, separated by high resolution gas chromatography, and measured by high resolution mass spectrometry. 2. Apparatus 2.1 Sampling. A schematic of the sampling train used in this method is shown in Figure 23-1. Sealing greases may not be used in assembling the train. The train is identical to that described in section 2.1 of Method 5 of this appendix with the following additions: Method 23 8/3/2017 2.1.1 Nozzle. The nozzle shall be made of nickel, nickel-plated stainless steel, quartz, or borosilicate glass. 2.1.2 Sample Transfer Lines. The sample transfer lines, if needed, shall be heat traced, heavy 1 1 walled TFE ( ⁄2 in. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

Wo 2007/086935 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 2 August 2007 (02.08.2007) WO 2007/086935 A2 (51) International Patent Classification: Not classified (US). MYERS, Eugene, W. [-/US]; 20 Commercial Street, Branford, CT 06405 (US). SIMPSON, John, (21) International Application Number: W. [US/US]; 23 Woodland Road, Suite B4, Madison, PCT/US2006/030235 CT 06443 (US). VOLKMER, Greg, A. [-/US]; 20 Commercial Street, Branford, CT 06405 (US). (22) International Filing Date: 1 August 2006 (01.08.2006) (74) Agent: HOPKINS, Brian, P.; Mintz, Levin, Cohn, Ferris, (25) Filing Language: English Glovsky And Popeo, Rc, Chrysler Center, 666 Third Av enue, New York, NY 10017 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 11/195,254 1 August 2005 (01.08.2005) US AT,AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, (71) Applicant (for all designated States except US): 454 LIFE GB, GD, GE, GH, GM, HN, HR, HU, ID, IL, IN, IS, JP, SCIENCES CORPORATION [US/US]; 20 Commercial KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, Street, Branford, CT 06405 (US). LU, LV,LY,MA, MD, MG, MK, MN, MW, MX, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, (72) Inventors; and SD, SE, SG, SK, SL, SM, SY, TJ, TM, TN, TR, TT, TZ, (75) Inventors/Applicants (for US only): MCDADE, Keith, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW E. -

I I I I Executive Summary
I I I CONTAMINANTS IN FISH AND SEDIMENTS OF THE GREAT SWAMP NATIONAL WILDLIFE REFUGE, MORRIS COUNTY, NEW JERSEY: I A 10-YEAR FOLLOW-UP INVESTIGATION DEC ID# 9950003.1 I ECDM Catalog # 5050048 I I I Prepared for: U.S. Fish & Wildlife Service Northeast Region Division of Refuges and Wildlife I Hadley, Massachusetts I Prepared by: I U.S. Fish & Wildlife Service New Jersey Field Office I Plem,antville, New Jersey I I August 2005 I Project Biologist: Clay M. Stem Assistant Supervisor: Timothy J. Kubiak I Supen,isor: Clifford G. Day I I 2 I I I I EXECUTIVE SUMMARY Located in Morris County, New Jersey about 25 miles west of New York City's Time Square, the I U.S. Fish & Wildlife Service's (Service) Great Swamp National Wildlife Refuge (GSNWR) encompasses approximately 7,500 acres ::,fwhich 3,660 acres are designated and managed as a National Wilderness Area. The GSNWR's wetlands provide important ecological functions, I including floodwater attenuation, groundwater recharge, pollution abatement, wildlife habitat, as well as recreational benefits for the public. In many portions of the Great Swamp watershed, significant areas of native soils have been disturbed by development to the extent that the I original soil profiles no longer exist. Development-related activities such as grading, infilling, and compaction have adversely altered the native soil's infiltration capacity and runoff potential I and thereby have increased storm-water 11ediment loading into many of the watershed's streams. The purpose of this investigation is to conduct a 10-year follow-up to a 1988 investigation (USFWS 1991) characterizing ambient concentrations of metals, organochlorines, and polycyclic I aromatic hydrocarbons (P AHs) in GSNWR sediments, and metals and organochlorine in fish inhabiting the GSNWR. -

DEKRA Process Safety Instrumentation Equipment Brochure
PROCESS SAFETY EQUIPMENT – Expertise in Explosion & Process Safety Full Instrument Index Carius Tube .......................................................................................... 6 Gas Burette .......................................................................................... 7 Minimum Ignition Energy (MIE) Cloud .......................................... 8 Minimum Ignition Temperature (MIT) Cloud ................................ 9 20 Litre Sphere ..................................................................................10 Portable Explosion Kit ......................................................................11 Group A/B Dust Flammability Lab Rig ........................................12 Layer Ignition Temperature .............................................................13 Burning Rate Apparatus ..................................................................14 Oxidising Solids Test Apparatus ...................................................15 Autoignition Temperature (AIT) ......................................................16 BAM Fallhammer .............................................................................17 BAM Friction......................................................................................18 Koenen Tube .....................................................................................19 Combined Time Pressure/Oxidising Liquids ...............................20 Floor Glove & Footwear Kit ...........................................................21 Liquid Conductivity -

OHAUS New Scout Balance Range
Catalogue OHAUS new Scout Balance Range Touchscreen Page 121 Memmert Ovens & Incubators New bollé German Made Page 140 Rush Plus Small Has Arrived Page 77 Schott-DURAN Youtility Bottles A New Age Page 6 SAVINGS Up to 70% off major brands Bacto Laboratories Feb 2018 BactoIssue Laboratories 7.5 Pty Ltd - Phone (02) 9823-9000Proudly - Email [email protected] Supplying - (prices Science excl GST) Since 1966 1 Contents A Centrifuge 145 Flask, Volumetric Glass 17 Alcohol Wipes 55 Centrifuge Tube, Glass 24 Flask, Volumetric Plastic 29 Analytical Balance 117 Centrifuge Tube, Plastic 44 Forceps 88 Aspirator Bottle, Plastic 42 Chemicals 67 Forceps, Artery 92 Autoclave Indicators 59 Clamps, Bosshead 96 Fridge Thermometers 102 Autoclave Tape 59 Clamps, Burettes 97 Funnel, Glass 15 Autoclave Waste Bags 61 Clamps, Retort 96 Funnel, Plastic 30 Clinical Centrifuge 145 G - H B Coats, Laboratory 74 Gloves, Examination 72 Bacticinerator 82 Cold Bricks 69 Gloves, Safety 71 Bags, Waste 61 Colony Counter 81 Gowns 75 Balance, Moisture 119 Conductivity Meter 109 Hand Wash, Alcohol 55 Balances 116 Conical Measure, Plastic 27 Hand Wash, Cleanser 56 Batteries 102 Contaminated Waste Bags 62 Hand Wash, Sanitiser 55 Beaker, Glass 11 Coplin Jar 53 Hockey Stick 50 Beaker, Plastic 27 Corrosive Cabinets 85 Hotplate 124 Bench Roll 58 Cover Slips, Glass 52 Hotplate Stirrer 124 Biju McCartney Bottle 25 Cryo Vial, Plastic 37 Hygrometers 102 Bins, Broken Glass 61 Culture Media 50 I Bins, Chemotherapy 65 Culture Tube, Glass 24 Incubator 138 Bins, Contaminated Waste 61 Cuvettes, -

Seit 1872 Produziert Usbeck Laborgeräte Aus Metall
Seit 1872 produziert Usbeck Laborgeräte aus Metall. Am Standort Radevormwald fertigt das familiengeführte Unternehmen ein breites Sortiment hochwertiger Laborartikel. Langlebigkeit, komfortable Handhabung und ansprechendes Design zeichnen Usbeck Produkte aus. Im Bestreben zur Nutzung nachhaltiger Fertigungsverfahren hat sich Usbeck auf die Verarbeitung von Zink spezialisiert. Im Druckgießverfahren entstehen Bauteile mit ausgezeichneter Oberflächenqualität, hoher Festigkeit und Härte. Geringer Energieverbrauch während der Produktion sowie die vollständige Recyclingfähigkeit machen Zink zum umweltfreundlichen Ausgangsmaterial unseres Stativprogramms. Die Sicherheit und die Erleichterung der täglichen Arbeit im Labor sind Leitfaden unserer Bemühungen. Zur Erreichung dieser Ziele stehen wir im regelmäßigen Dialog mit den Anwendern. Since its foundation in 1872 Usbeck produces laboratory metalware. At the manufacturing facility in Radevormwald the family owned business produces a broad range of high quality laboratory equipment. Longevity, convenient handling and attractive design characterize Usbeck products. With the objective to employ sustainable production techniques, Usbeck has specialized in zinc die casting, creating components with excellent surface quality, high rigidity and hardness. Low energy consumption during production as well as full recyclability make zinc the suitable starting material of our scaffolding. Safety and facilitation of daily laboratory work are the guidelines of our efforts. To achieve these objectives, we continuously -

(12) United States Patent (10) Patent No.: US 8,557,518 B2 Jovanovich Et Al
US008557518B2 (12) United States Patent (10) Patent No.: US 8,557,518 B2 Jovanovich et al. (45) Date of Patent: Oct. 15, 2013 (54) MCROFLUDIC AND NANOFLUIDC (56) References Cited DEVICES, SYSTEMS, AND APPLICATIONS U.S. PATENT DOCUMENTS (75) Inventors: Stevan Bogdan Jovanovich, Livermore, 3,190,310 A 6/1965 Honsinger CA (US); Iuliu I. Blaga, Fremont, CA 11/1967 Ando et al. (US); Michael Nguyen, San Jose, CA 3,352,643 A (US); William D. Nielsen, San Jose, CA (Continued) (US); Mattias Vangbo, Fremont, CA (US) FOREIGN PATENT DOCUMENTS CA 2433145 A1 5, 2002 (73) Assignee: IntegenX Inc., Pleasanton, CA (US) EP O459.241 B1 12, 1991 (*) Notice: Subject to any disclaimer, the term of this (Continued) patent is extended or adjusted under 35 OTHER PUBLICATIONS U.S.C. 154(b) by 109 days. Shaikh et all “A modular microfluidic archetecture for integrated (21) Appl. No.: 12/845,650 biochemical analysis”, PNAS, 2005, 102: 9745-9750.* (22) Filed: Jul. 28, 2010 (Continued) (65) Prior Publication Data Primary Examiner — Betty Forman US 2012/O1151.89 A1 May 10, 2012 (57) ABSTRACT Related U.S. Application Data The present invention discloses the integration of program (63) Continuation of application No. 12/526,015, filed as mable microfluidic circuits to achieve practical applications application No. PCT/US2008/053099 on Feb. 5, 2008. to process biochemical and chemical reactions and to inte grate these reactions. In some embodiments workflows for (60) Provisional application No. 60/899,630, filed on Feb. biochemical reactions or chemical workflows are combined. 5, 2007. Microvalves such as programmable microfluidic circuit with Y valves and flow through valves are disclosed. -
Science Udyog SCIENCE UDYOG 2767 , TIMBER MARKET Ambala Cantt - 133001 Haryana - INDIA +91 9671438625 ,8397904611 E-Mail: [email protected]
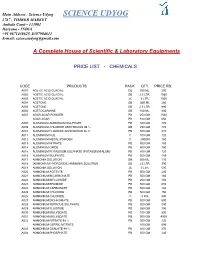
Main Address : Science Udyog SCIENCE UDYOG 2767 , TIMBER MARKET Ambala Cantt - 133001 Haryana - INDIA +91 9671438625 ,8397904611 E-mail: [email protected] A Complete House of Scientific & Laboratory Equipments PRICE LIST - CHEMICALS CODE PRODUCTS PACK QTY. PRICE RS. A001 ACETIC ACID GLACIAL GB 500 ML. 200 A002 ACETIC ACID GLACIAL GB 2.5 LTR. 1080 A003 ACETIC ACID GLACIAL JC 5 LTR. 1500 A004 ACETONE GB 500 ML 260 A005 ACETONE GB 2.5 LTR. 990 A006 ACETOCARMINE GB 100 ML. 800 A007 AGAR AGAR POWDER PB 250 GM 1500 AGAR AGAR PB 100 GM 650 A008 ALUMINIUM AMMONIUM SULPHATE PB 500 GM 170 A009 ALUMINIUM CHLORIDE ANHYDROUS 98 % GB 250 GM 170 A010 ALUMINIUM FLUORIDE ANHYDROUS 96 % PB 500 GM 410 A011 ALUMINIUM FOIL C 100 GM 120 A012 ALUMINIUM METAL POWDER C 250GM 180 A013 ALUMINIUM NITRATE PB 500 GM 180 A014 ALUMINIUM OXIDE PB 500 GM 150 A015 ALUMINIUM POTASSIUM SULPHATE (POTASSIUM ALUM) PB 500 GM 120 A016 ALUMINIUM SULPHATE PB 500 GM 148 A017 AMMONIA SOLUTION GB 500 ML. 110 A018 (AMMONIUM HYDROXIDE) AMMONIA SOLUTION GB 2.5 LTR. 390 A019 AMMONIA SOLUTION JC 5 LTR. 590 A020 AMMONIUM ACETATE PB 500 GM 240 A021 AMMONIUM BICARBONATE PB 500 GM 180 A022 AMMONIUM BIFLUORIDE PB 250 GM 156 A023 AMMONIUM BROMIDE PB 500 GM 680 A024 AMMONIUM CARBONATE PB 500 GM 194 A025 AMMONIUM CHLORIDE PB 500 GM 150 A026 AMMONIUM CHLORIDE C 5 KG. 990 A027 AMMONIUM DICHROMATE PB 500 GM 690 A028 AMMONIUM FERROUS SULPHATE PB 500 GM 240 A029 AMMONIUM FLUORIDE PB 250 GM 196 A030 AMMONIUM MOLYBDATE PB 100 GM 890 A031 AMMONIUM MOLYBDATE PB 500 GM 4500 A032 AMMONIUM NITRATE 98 % PB 500 GM 136 AMMONIUM CERRIC NITRATE 100 GM 595 A033 AMMONIUM OXALATE 99 % PB 500 GM 270 A034 AMMONIUM PHOSPHATE PB 500 GM 240 A035 AMMONIUM SULPHATE PB 500GM 142 A036 AMYL ACETATE ISO GB 500 ML.