• the Public Health Problem • the National Academies Response
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 United States District Court for the Southern District
Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 1 of 167 UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF IOWA, CENTRAL DIVISION THE STATE OF IOWA, Plaintiff, v. ABBOTT LABORATORIES, INC., AGOURON PHARMACEUTICALS, INC., JURY TRIAL REQUESTED ALPHARMA, INC., ALZA CORPORATION, AMGEN, INC., ASTRAZENECA L.P., ASTRAZENECA PHARMACEUTICALS, LP., AVENTIS BEHRING L.L.C., COMPLAINT BARR LABORATORIES, INC., BAXTER INTERNATIONAL, INC., BAXTER HEALTHCARE CORPORATION, BAYER CORPORATION, BAYER PHARMACEUTICALS CORPORATION, BEN VENUE LABORATORIES, INC., BOEHRINGER INGELHEIM CORPORATION, BOEHRINGER INGELHEIM PHARMACEUTICALS, INC., BRISTOL-MYERS SQUIBB COMPANY, CENTOCOR, INC., CHIRON CORPORATION, DERMIK LABORATORIES, INC., DEY, INC., DEY, L.P., ELI LILLY AND COMPANY, EMD, INC., ENDO PHARMACEUTICALS, INC., ETHEX CORPORATION, ETHICON, INC., FOREST LABORATORIES, INC., FOREST PHARMACEUTICALS, INC. GENEVA PHARMACEUTICALS, GLAXOSMITHKLINE, PLC, GLAXOWELLCOME, INC., GREENSTONE, LTD., HOECHEST MARION ROIUSSEL, INC., HOFFMAN-LAROCHE, INC., 1 Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 2 of 167 IMMUNEX CORPORATION, IVAX CORPORATION, IVAX PHARMACEUTICALS, INC., JANSSEN PHARMACEUTICA PRODUCTS, LP, JOHNSON & JOHNSON, KING PHARMACEUTICALS, INC., KING RESEARCH AND DEVELOPMENT, MCNEIL-PPC, INC., MEDIMMUNE, INC., MERCK & CO., INC., MONARCH PHARMACEUTICALS, INC., MYLAN LABORATORIES, INC., MYLAN PHARMACEUTICALS, INC., NOVARTIS PHARMACEUTICALS CORPORATION, NOVOPHARM USA, INC., ONCOLOGY THERAPEUTICS NETWORK CORP., ORTHO-MCNEIL PHARMACEUTICAL, INC., -

Update Covers C1-C4 Iss2 2005.Indd
In Compliance and In Court by John B. Reiss, Ph.D., and William M. Janssen his article examines various fraud and abuse cases is the simultane- violated the Anti-Kickback Statute. Tlegal issues facing the medical ous fi ling of False Claims Act charges. Under the government’s theory, these device, pharmaceutical, and biologics Because the False Claims Act permits inducements resulted in an illegal use industries; court decisions or settle- fi nes of up to three times the amount of the drugs, and claims fi led for these ments over the past year; and suggested of the claim—and penalties of between uses were thus false. Also brought actions. New guidelines set standards $5,500 and $11,000 per claim—penal- into focus by this case were the False that industry leaders should consider as ties can add up quickly to billions of Claims Act’s qui tam provisions, which they plan product development, sales, dollars, giving the government an enor- allow whistleblowers to participate in and marketing strategies. mous “hammer” with which to exact up to 25% of the government’s recov- settlements. ery. A majority of the recent cases have Fraud and Abuse and the The complexity of the application resulted from whistleblower fi lings. False Claims Act of these laws is demonstrated by the TAP Pharmaceuticals made an Fraud and abuse issues dominated Warner-Lambert case.1 The primary $875 million payment in October 2001, the headlines during 2004. The U.S. allegation involved the company’s partly as the result of its guilty plea for Attorney and the Department of Health off-label marketing of its epilepsy violating the Prescription Drug Market- and Human Services (DHHS) Offi ce of drug, Neurontin®, in violation of the ing Act.2 The government also alleged Inspector General (OIG) continued a Federal Food, Drug, and Cosmetic Act manipulation of the average wholesale high level of activity, and states’ attor- (FDCA). -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -
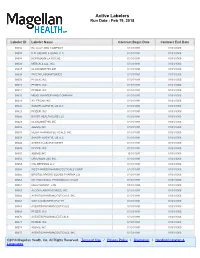
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Bioscience Industry Companies This List Includes Georgia Companies That Fall Into the Bioscience Industry, with a Brief Description Shown
Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Company Name City Description Website Develops novel automated technology for the 3 Ti (Transfusion Transplantation Technologies) Atlanta diagnostic testing of blood associated with blood http://www.3tibio.com/location.html transfusions. Develops 3-D modeling technology for dental 360 Imaging Atlanta http://www.360imaging.com surgeries. Develops real-time 3-D visualization technology for 3D SURGICAL SOLUTIONS Marietta minimally invasive surgeries. Develops and manufactures skin-safe tape for 3M Atlanta healthcare. http://www.3m.com 3R Gloves Roswell Manufactures reusable and recyclable latex gloves. 4P Therapeutics Norcross Manufacturer of drug delivery technologies www.4ptherapeutics.com Manufactures blood pressure monitors, scales, A&D Engineering Duluth www.andonline.com thermometers, and activity monitors. Manufactures and specializes in the production of A&L Shielding Rome www.alshielding.com radiation shielding products. Develops and produces diamond and carbide A&M INSTRUMENTS Alpharetta www.aminstr.com cutting tools. Manufactures hospital air purification equipment Abatement Technologies Suwanee www.abatement.com for infection control. Science based offerings in diagnostics, medical Abbott Laboratories Alpharetta www.abbott.com devices, nutrition, and pharmaceuticals. Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Develops proprietary technology to discover Abeome Athens www.abeomecorp.com therapeutic and diagnostic monoclonal antibodies. Manufactures medical devices and provides Accellent Trenton research for several non-medical industries. Savannah and Augusta Testing services for pharmaceutical and Acuren Inspection www.acuren.com Metro biotechnology companies. Pharmaceutical research focused on cell Aderans Pharmaceutical Research Institute Marietta engineering solutions for hair loss. -

Women's Health Disparities Vary by Ethnic Group
86 Practice Trends FAMILY P RACTICE N EWS • March 15, 2005 Women’s Health Disparities Vary by Ethnic Group BY JOYCE FRIEDEN there’s not much analysis of [health data care. They also have the highest mortali- their leisure time, “which is very impor- Associate Editor, Practice Trends on] racial and ethnic groups by gender.” ty rates for coronary heart disease, stroke, tant for obesity issues.” To further examine the issue, the cen- and diabetes, and the highest incidence of American Indian and Alaskan Native WASHINGTON — More programs need ter analyzed data and published the results AIDS and lung cancer. women had the second-lowest morality to be developed to address the specific in a report titled, “Making the Grade on Latinas have the lowest mortality rate rate from stroke, but they fared worst of health needs of minority women, Elena Women’s Health,” that outlines disparities from stroke but are the second-least like- all groups for smoking, binge drinking, Cohen said at the annual meeting of the in women’s health care in different states. ly group to be screened for cervical can- mortality from cirrhosis, and violence American Public Health Association. For example, black women have the cer, and they fare worse in cervical cancer against them, Ms. Cohen said. “Racial minorities are projected to make highest rate of Pap smears and the lowest incidence and mortality, she said. This “The Asian American/Pacific Islander up almost half the population by 2050,” rate of osteoporosis, but also have the group has the highest percentage of unin- group fared best in preventive health be- said Ms. -

Disclosures ASCP Annual Meeting FAIRMONT SCOTTSDALE PRINCESS MAY 30-JUNE 3, 2016
Disclosures ASCP Annual Meeting FAIRMONT SCOTTSDALE PRINCESS MAY 30-JUNE 3, 2016 www.ASCPP.org The following presenters have something to disclose: Aaronson, Scott: Neuronetics, Research support, Consulting – Self; Sunovion, Speaker – Self; Otsuka/Lundbeck, Speaker – Self; Genomind, Scientific Advisory Board - Self Achtyes, Eric: Avanir, Research grant, travel - Self; Otsuka, Research grant, travel - Self; Janssen, Research grant, travel - Self; Vanguard Research Group, Research grant, travel, advisory board - Self Alphs, Larry: Janssen, Employee - Self; Johnson & Johnson, Stockholder - Self Althof, Stanley: Abbott, Research support or consulting fees - Self; Allergan, Research support or consulting fees - Self; Astellas, Research support or consulting fees - Self; Eli Lilly, Research support or consulting fees - Self; Evidera, Research support or consulting fees - Self; Ixchelsis, Research support or consulting fees - Self; Palatin Technologies, Inc, Research support or consulting fees - Self; Pfizer, Research support or consulting fees - Self; Promescent, Research support or consulting fees - Self; Sprout Pharmaceuticals, Research support or consulting fees - Self; S1 Biopharmaceuticals, Research support or consulting fees - Self; Strategic Science Technologies, Research support or consulting fees - Self; Trimel Pharmaceuticals, Inc, Research support or consulting fees - Self; Vyrix, Research support or consulting fees - Self Anton, Raymond: Lilly, Supporter of ACTIVE - Self; Lundbeck, Supporter of ACTIVE - Self; Abbvie, Supporter of -

Biopharmaceutical Industry Speakers List Cost and Value of Medicines
Biopharmaceutical Industry Speakers List Cost and Value of Medicines PHARMACEUTICAL RESEARCH AND MANUFACTURERS OF AMERICA About the Speakers List The Biopharmaceutical Industry Speakers List contains names of executives at the Pharmaceutical Research and Manufacturers of America (PhRMA) and its member companies available to participate in events and panel discussions on the cost and value of prescription medicines. A photo, biography and contact information is available for each speaker. There is no fee associated with any speaking engagement. Parties interested in securing a speaker should contact these individuals directly to discuss their participation. PhRMA and its member companies will attempt to accommodate all requests, but cannot guarantee participation. For questions or more information, please contact the PhRMA Newsroom at [email protected] or call 202-835-3460. BIOPHARMACEUTICAL INDUSTRY SPEAKERS LIST 2 Available Speakers PhRMA JEFFREY BOND LORI M. REILLY, J.D. Senior Vice President, Advocacy Executive Vice President, Policy and Research Washington, D.C. Washington, D.C. JENNIFER BRYANT JAMES “MIT” M. SPEARS, J.D. Senior Vice President, Policy and Research General Counsel, Washington, D.C. Executive Vice President, Legal Washington, D.C. RANDY BURKHOLDER Vice President, Policy and Research STEPHEN J. UBL Washington, D.C. President and Chief Executive Officer Washington, D.C. WILLIAM “BILL” CHIN, M.D. Chief Medical Officer and Executive Vice MICHAEL YBARRA, M.D. President, Science and Regulatory Affairs Senior Director, Alliance Development Washington, D.C. Washington, D.C. JOSEPHINE “JOSIE” C. MARTIN ROBERT ZIRKELBACH Executive Vice President, Public Affairs Senior Vice President, Communications Washington, D.C. Washington, D.C. PhRMA Member Companies AbbVie LATIF AKINTADE, M.D., MBA JEFFREY R. -

The Food and Drug Administration on February 16-18, 2005 Held an Advisory Committee Meeting to Discuss the Cardiovascular Risk P
The Food and Drug Administration on February 16-18, 2005 held an advisory committee meeting to discuss the cardiovascular risk posed by painkillers known as Cox- 2 inhibitors, which include Celebrex, Bextra and Vioxx. The former two drugs are manufactured by Pfizer. The latter is manufactured by Merck. Novartis also has a Cox-2 inhibitor in its pipeline. At the end of the hearing, the FDA advisory panel voted to keep all three on the market, though with heightened warnings about the dangers posed by this class of drugs. At the request of the New York Times, the Center for Science in the Public Interest evaluated the 32 scientific experts chosen by the FDA to evaluate these drugs. The CSPI research uncovered affiliations between 10 of the scientists that served on the committee and the three manufacturers of Cox-2 inhibitors. This would appear to be a direct violation of the Federal Advisory Committee Act, which prohibits scientists with direct conflicts of interest from serving on panels offering advise to federal regulatory agencies. Another 17 scientists had other ties to drug manufacturers, though not the three with products under consideration at the meeting. According to a New York Times analysis of the votes, the advisory committee would have voted against Bextra and Vioxx staying on the market had scientists with conflicts of interest been excluded from the vote. Here is the CSPI analysis of the FDA Advisory Panel: CSPI found ten (10) physician/researchers with direct ties to Pfizer, Merck or Novartis (including G.D. Searle and Pharmacia, which are now part of Pfizer). -

Strategic Report for Abbott Laboratories
Strategic Report for Abbott Laboratories Edward Wexler-Beron John Bejjani Mark Melief April 12, 2006 Table of Contents Executive Summary .......................................................................... 2 Company Background ....................................................................... 4 Important Contributions and Product Developments.................................... 4 Growth, Mergers/Acquisitions and a Spin Off ............................................. 5 Setbacks and Litigation Throughout the 20th Century ................................... 7 Competitive Analysis ........................................................................ 8 Internal Rivalry .................................................................................. 9 Entry ............................................................................................. 10 Substitutes and Complements .............................................................. 11 Buyer Power .................................................................................... 12 Supplier Power................................................................................. 14 Financial Analysis ........................................................................... 14 Segment-By-Segment Analysis............................................................. 16 ROA Analysis by Segment ................................................................... 20 Margin Analysis ................................................................................ 21 DuPont -

89Th Annual Meeting
EXHIBITOR INFORMATION Refunds and Cancellations Cancellations received in writing by June 12, 2020 will be subject to a 25% administrative fee. There will be no refunds for cancellations received after June 12th. Conducting Exhibits Drawings, raffles, or quiz-type contests will be permitted, but must be submitted to show management for final approval. Electrical or other mechanical apparatus must be muffled so noise does not interfere with other exhibitors. Character of the exhibits is subject to approval of the Section. The right is reserved to refuse applications of concerns not meeting standards required or expected, as well as the right to curtail exhibits or parts of exhibits that reflect against the character of the meeting. This applies to displays, literature, advertising, novelties, souvenirs, conduct of persons, etc. Space Assignment * Space assignment will be given to companies in the order in which applications are received. Following the June 12, 2020 deadline, exhibit assignments will be made on a space available basis. If space is filled by June 12, 2020, applications received will be placed on a waiting list and notified immediately. Exhibitors wishing to avoid assignment of space adjacent to a particular company should indicate this on their application. Careful consideration will be given to such requests*. *The New England Section, AUA reserves the right to alter the Exhibit Floor Plan at any time. Installation The exhibit hall will be available for set-up from 9:00 a.m. - 2:30 p.m. on Thursday, September 10, 2020. All exhibits must be set by 2:30 p.m. without exception. Assembly of exhibits during regularly scheduled exhibit hours will not be permitted. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC.