Notice of Potential Labeler Terminations
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

BIOWORLD TODAY Inquiry
BIOWORLDTM TODAY THE DAILY BIOPHARMACEUTICAL NEWS SOURCE JUNE 30 , 2016 BIOTECH’S MOST RESPECTED NEWS SOURCE FOR MORE THAN 20 YEARS VOLUME 27, NO. 126 LIPID JAM NOT OVER EASILY STOPPED FOR FUTILITY Waffle house? FDA Galena Biopharma implodes as PRESENT review fidgets endpoint, puts cancer vaccine Neuvax future in doubt outcome details; By Jennifer Boggs, Managing Editor Esperion grilled on holdup With the bulk of Galena Biopharma Inc.’s value riding on cancer vaccine Neuvax By Randy Osborne, Staff Writer (nelipepimut-S), the firm’s shares predictably plunged to a new 52-week low Wednesday as an independent data monitoring committee (IDMC) recommended the Analysts had plenty of questions but PRESENT phase III study in breast cancer be stopped for futility following a planned Esperion Therapeutics Inc. offered few interim analysis. But it’s the troubling language in the IDMC’s letter, suggesting the answers regarding the FDA’s stalling on placebo arm might actually have bested the treatment arm, that could signal the end oral, once-daily bempedoic acid (ETC- of the road for Neuvax. 1002) for lipid lowering, after the agency See Galena, page 3 See Esperion, page 4 CHINA DEALS AND M&A IN THE CLINIC Pfizer invests in Asia Merck strikes cancer SUPER ‘NOVA’ with $350M biotech vaccines deal with Tesaro shares blast plant in Hangzhou Moderna, delivering off as niraparib hits By Haky Moon, Staff Writer $200M up front PFS in ovarian cancer HONG KONG – China’s economy may By Michael Fitzhugh, Staff Writer By Marie Powers, News Editor be slowing down, but multinationals Findings from the phase III NOVA trial are positioning themselves to leverage Cancer vaccines tailored to fit tumor- specific profiles are at the heart of a new of niraparib in women with recurrent it as best they can while navigating a ovarian cancer blasted shares of still-complex regulatory environment. -

Monday, April 22 Chicago Bears Room Chicago Bulls Room Chicago Cubs Room Merck KLOX Technologies Immune Design Leading Biote
As of 4/23/2013 Schedule subject to change Monday, Chicago Bears Room Chicago Bulls Room Chicago Cubs Room April 22 Merck KLOX Technologies Immune Design 1:00 PM Leading Biotech/Big Pharma Medical Devices Vaccines Eli Lilly NewSouth Innovations Syntiron 1:15 PM Leading Biotech/Big Pharma University/Academia Vaccines Amgen Radius Health BioCrea 1:30 PM Leading Biotech/Big Pharma Musculoskeletal Neurology/CNS Nat. Inst. of Neurological Dis. & Stroke Cytokinetics Xenon Pharmaceuticals 1:45 PM Neurology/CNS Musculoskeletal Neurology/CNS Curis OrgaNext Research BV Trigemina 2:00 PM Oncology Regenerative Medicine Neurology/CNS Verastem Flexion Therapeutics Neurocrine Biosciences 2:15 PM Oncology Musculoskeletal Hormone Therapy/CNS Michael J. Fox Foundation Antisense Pharma GmbH Versartis 2:30 PM Non-profit/Patient Advocacy Oncology Hormone Therapy Takeda Pharmaceutical Company TBD KODE Biotech 2:45 PM Leading Biotech/Big Pharma Drug Delivery Resverlogix Corp. Advaxis Q Chip 3:00 PM Cardiovascular Disease Oncology Drug Delivery Grünenthal GmbH Array BioPharma 3:15 PM Neurology/CNS Oncology/Drug Discovery Discovery Labs Mersana Therapeutics 3:30 PM Drug Delivery/Pulmonary Oncology Bayer HealthCare Igenica 3:45 PM Leading Biotech/Big Pharma Oncology Presentations are open to all Convention attendees and are located outside the main entrance of the BIO Business Forum As of 4/23/2013 - Schedule subject to change Tuesday, Chicago Bears Room Chicago Bulls Room Chicago Cubs Room Chicago Blackhawks Room April 23 Pfizer 8:00 AM Leading Biotech/Big Pharma -

Medicaid System (Mmis) Illinois Department of Run Date: 08/08/2015 Provider Subsystem Healthcare and Family Services Run Time: 21:25:58 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 08/08/2015 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 21:25:58 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 10/01/2015 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 65162 AMNEAL PHARMACEUTICALS LLC 00074 ABBOTT LABORATORIES 69238 AMNEAL PHARMACEUTICALS, LLC 68817 ABRAXIS BIOSCIENCE, LLC 53150 AMNEAL-AGILA, LLC 16729 ACCORD HEALTHCARE INCORPORATED 00548 AMPHASTAR PHARMACEUTICALS, INC. 42192 ACELLA PHARMACEUTICALS, LLC 66780 AMYLIN PHARMACEUTICALS, INC. 10144 ACORDA THERAPEUTICS, INC. 55724 ANACOR PHARMACEUTICALS 00472 ACTAVIS 10370 ANCHEN PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 62559 ANIP ACQUISITION COMPANY 45963 ACTAVIS INC. 54436 ANTARES PHARMA, INC. 46987 ACTAVIS KADIAN LLC 52609 APO-PHARMA USA, INC. 49687 ACTAVIS KADIAN LLC 60505 APOTEX CORP. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 63323 APP PHARMACEUTICALS, LLC. 67767 ACTAVIS SOUTH ATLANTIC 42865 APTALIS PHARMA US, INC 66215 ACTELION PHARMACEUTICALS U.S., INC. 58914 APTALIS PHARMA US, INC. 52244 ACTIENT PHARMACEUTICALS 13310 AR SCIENTIFIC, INC. 75989 ACTON PHARMACEUTICALS 08221 ARBOR PHARM IRELAND LIMITED 76431 AEGERION PHARMACEUTICALS, INC. 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 50102 AFAXYS, INC. 24338 ARBOR PHARMACEUTICALS, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 59923 AREVA PHARMACEUTICALS 27241 AJANTA PHARMA LIMITED 76189 ARIAD PHARMACEUTICALS, INC. 17478 AKORN INC 24486 ARISTOS PHARMACEUTICALS, INC. 24090 AKRIMAX PHARMACEUTICALS LLC 67877 ASCEND LABORATORIES, L.L.C. 68220 ALAVEN PHARMACEUTICAL, LLC 76388 ASPEN GLOBAL INC. 00065 ALCON LABORATORIES, INC. 51248 ASTELLAS 00998 ALCON LABORATORIES, INC. 00469 ASTELLAS PHARMA US, INC. 25682 ALEXION PHARMACEUTICALS 00186 ASTRAZENECA LP 68611 ALIMERA SCIENCES, INC. -

Galena Biopharma, Inc. (Exact Name of Registrant As Specified in Its Charter) ______
ANNUAL REPORT 201 OUR MISSION GALENA BIOPHARMA DEVELOPS AND COMMERCIALIZES INNOVATIVE, TARGETED ONCOLOGY TREATMENTS THAT ADDRESS MAJOR UNMET MEDICAL NEEDS TO ADVANCE CANCER CARE. MANAGEMENT TEAM Mark J. Ahn, Ph.D., President & Chief Executive Officer Remy Bernarda, Vice President, Marketing & Communications Gavin Choy, Pharm.D., Senior Vice President, Clinical Operations & Cinical Science Ryan Dunlap, CPA, Vice President, Chief Financial Officer Brian Hamilton, M.D., Ph.D., Executive Vice President & Chief Medical Officer Robert Laliberte, MS, Vice President, Clinical Operations & Clinical Science Christopher Lento, Vice President, Sales & Commercial Operations Hana B. Moran, Ph.D., Senior Vice President, Regulatory & Compliance Patricia Murphy, Vice President, Regulatory Affairs & Compliance Mark W. Schwartz, Ph.D., Executive Vice President & Chief Operating Officer SCIENTIFIC ADVISORY BOARD COL George Peoples, M.D., F.A.C.S., Chief, Surgical Oncology, Brooke Army Medical Center; Director and Principal Investigator, Cancer Vaccine Development Program, San Antonio Military Medical Center Hope S. Rugo, M.D., Clinical Professor of Medicine, Division of Hematology and Oncology at the University of California San Francisco Helen Diller Family Comprehensive Cancer Care Center Robert Figlin, M.D., F.A.C.P., Dr. Figlin is a fellow of the American College of Physicians and the International Society for Biologic Therapy as well as a member of the American Society of Clinical Oncology, the American Association for Cancer Research, and the American Urological Association. Dr. Gabriel N. Hortobagyi, M.D., F.A.C.P., is a Professor of Medicine and Chairman of the Department of Breast Medical Oncology and holds the Nellie B. Connally Chair in Breast Cancer Research at the University of Texas MD Anderson Cancer Center (MDACC). -

Biotech Watchlist 2013 Update: Exciting Ideas Have Explosive Potential
Biotech Watchlist 2013 Update: Exciting Ideas Have Explosive Potential The Life Sciences Report www.TheLifeSciencesReport.com 05/02/2013 In January 2013, The Life Sciences Report debuted its biotech COMPANIES MENTIONED Watchlist, which outlined ideas for investors keyed to catalysts in Amarin Corp. the drug development process that typically move biotechnology Astellas Pharma Inc. stocks. All stocks are affected by catalysts, but nowhere do they Bayer provide more leverage (or deleverage) than in biotech. We Cardinal Health Inc. predicted the new year would present legitimate prospects for Celsion Corp. portfolio growth. So far, that has been the case. Although the party has given Galena Biopharma Inc. way to some fatigue, the punch bowl is still on the table and the biotech Hyperion Therapeutics upswing continues. Inc. Medivation Inc. Source: George S. Mack of The Life Sciences Report Navidea Biopharmaceuticals Inc. Back in January, our friends and collaborators at San Diego-based Sagient Novartis AG Research, publishers of the BioMedTracker, offered important information about Onyx Pharmaceuticals market-moving data and events that can make or break smaller companies. In Inc. addition, a group of key biotech analysts weighed in on their best ideas—17 stocks Peregrine Pharmaceuticals Inc. in all. It was the perfect storm of individual ideas combined with a biotech wind from Pfizer Inc. 2012 still billowing the sails. As of April 22, the NASDAQ Biotechnology (NBI) Pharmacyclics Inc. index is up 26.6%. Have the biotech companies that made inaugural Biotech Prana Biotechnology Ltd. Watchlist—and their stocks—met the targets identified in the first quarter of 2013? Sangamo BioSciences Are there new catalysts? Check out the progress in the updated April 2013 Biotech Inc. -

1 United States District Court for the Southern District
Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 1 of 167 UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF IOWA, CENTRAL DIVISION THE STATE OF IOWA, Plaintiff, v. ABBOTT LABORATORIES, INC., AGOURON PHARMACEUTICALS, INC., JURY TRIAL REQUESTED ALPHARMA, INC., ALZA CORPORATION, AMGEN, INC., ASTRAZENECA L.P., ASTRAZENECA PHARMACEUTICALS, LP., AVENTIS BEHRING L.L.C., COMPLAINT BARR LABORATORIES, INC., BAXTER INTERNATIONAL, INC., BAXTER HEALTHCARE CORPORATION, BAYER CORPORATION, BAYER PHARMACEUTICALS CORPORATION, BEN VENUE LABORATORIES, INC., BOEHRINGER INGELHEIM CORPORATION, BOEHRINGER INGELHEIM PHARMACEUTICALS, INC., BRISTOL-MYERS SQUIBB COMPANY, CENTOCOR, INC., CHIRON CORPORATION, DERMIK LABORATORIES, INC., DEY, INC., DEY, L.P., ELI LILLY AND COMPANY, EMD, INC., ENDO PHARMACEUTICALS, INC., ETHEX CORPORATION, ETHICON, INC., FOREST LABORATORIES, INC., FOREST PHARMACEUTICALS, INC. GENEVA PHARMACEUTICALS, GLAXOSMITHKLINE, PLC, GLAXOWELLCOME, INC., GREENSTONE, LTD., HOECHEST MARION ROIUSSEL, INC., HOFFMAN-LAROCHE, INC., 1 Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 2 of 167 IMMUNEX CORPORATION, IVAX CORPORATION, IVAX PHARMACEUTICALS, INC., JANSSEN PHARMACEUTICA PRODUCTS, LP, JOHNSON & JOHNSON, KING PHARMACEUTICALS, INC., KING RESEARCH AND DEVELOPMENT, MCNEIL-PPC, INC., MEDIMMUNE, INC., MERCK & CO., INC., MONARCH PHARMACEUTICALS, INC., MYLAN LABORATORIES, INC., MYLAN PHARMACEUTICALS, INC., NOVARTIS PHARMACEUTICALS CORPORATION, NOVOPHARM USA, INC., ONCOLOGY THERAPEUTICS NETWORK CORP., ORTHO-MCNEIL PHARMACEUTICAL, INC., -

Update Covers C1-C4 Iss2 2005.Indd
In Compliance and In Court by John B. Reiss, Ph.D., and William M. Janssen his article examines various fraud and abuse cases is the simultane- violated the Anti-Kickback Statute. Tlegal issues facing the medical ous fi ling of False Claims Act charges. Under the government’s theory, these device, pharmaceutical, and biologics Because the False Claims Act permits inducements resulted in an illegal use industries; court decisions or settle- fi nes of up to three times the amount of the drugs, and claims fi led for these ments over the past year; and suggested of the claim—and penalties of between uses were thus false. Also brought actions. New guidelines set standards $5,500 and $11,000 per claim—penal- into focus by this case were the False that industry leaders should consider as ties can add up quickly to billions of Claims Act’s qui tam provisions, which they plan product development, sales, dollars, giving the government an enor- allow whistleblowers to participate in and marketing strategies. mous “hammer” with which to exact up to 25% of the government’s recov- settlements. ery. A majority of the recent cases have Fraud and Abuse and the The complexity of the application resulted from whistleblower fi lings. False Claims Act of these laws is demonstrated by the TAP Pharmaceuticals made an Fraud and abuse issues dominated Warner-Lambert case.1 The primary $875 million payment in October 2001, the headlines during 2004. The U.S. allegation involved the company’s partly as the result of its guilty plea for Attorney and the Department of Health off-label marketing of its epilepsy violating the Prescription Drug Market- and Human Services (DHHS) Offi ce of drug, Neurontin®, in violation of the ing Act.2 The government also alleged Inspector General (OIG) continued a Federal Food, Drug, and Cosmetic Act manipulation of the average wholesale high level of activity, and states’ attor- (FDCA). -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -
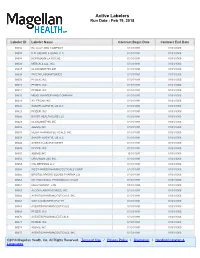
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Bioscience Industry Companies This List Includes Georgia Companies That Fall Into the Bioscience Industry, with a Brief Description Shown
Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Company Name City Description Website Develops novel automated technology for the 3 Ti (Transfusion Transplantation Technologies) Atlanta diagnostic testing of blood associated with blood http://www.3tibio.com/location.html transfusions. Develops 3-D modeling technology for dental 360 Imaging Atlanta http://www.360imaging.com surgeries. Develops real-time 3-D visualization technology for 3D SURGICAL SOLUTIONS Marietta minimally invasive surgeries. Develops and manufactures skin-safe tape for 3M Atlanta healthcare. http://www.3m.com 3R Gloves Roswell Manufactures reusable and recyclable latex gloves. 4P Therapeutics Norcross Manufacturer of drug delivery technologies www.4ptherapeutics.com Manufactures blood pressure monitors, scales, A&D Engineering Duluth www.andonline.com thermometers, and activity monitors. Manufactures and specializes in the production of A&L Shielding Rome www.alshielding.com radiation shielding products. Develops and produces diamond and carbide A&M INSTRUMENTS Alpharetta www.aminstr.com cutting tools. Manufactures hospital air purification equipment Abatement Technologies Suwanee www.abatement.com for infection control. Science based offerings in diagnostics, medical Abbott Laboratories Alpharetta www.abbott.com devices, nutrition, and pharmaceuticals. Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Develops proprietary technology to discover Abeome Athens www.abeomecorp.com therapeutic and diagnostic monoclonal antibodies. Manufactures medical devices and provides Accellent Trenton research for several non-medical industries. Savannah and Augusta Testing services for pharmaceutical and Acuren Inspection www.acuren.com Metro biotechnology companies. Pharmaceutical research focused on cell Aderans Pharmaceutical Research Institute Marietta engineering solutions for hair loss. -

Women's Health Disparities Vary by Ethnic Group
86 Practice Trends FAMILY P RACTICE N EWS • March 15, 2005 Women’s Health Disparities Vary by Ethnic Group BY JOYCE FRIEDEN there’s not much analysis of [health data care. They also have the highest mortali- their leisure time, “which is very impor- Associate Editor, Practice Trends on] racial and ethnic groups by gender.” ty rates for coronary heart disease, stroke, tant for obesity issues.” To further examine the issue, the cen- and diabetes, and the highest incidence of American Indian and Alaskan Native WASHINGTON — More programs need ter analyzed data and published the results AIDS and lung cancer. women had the second-lowest morality to be developed to address the specific in a report titled, “Making the Grade on Latinas have the lowest mortality rate rate from stroke, but they fared worst of health needs of minority women, Elena Women’s Health,” that outlines disparities from stroke but are the second-least like- all groups for smoking, binge drinking, Cohen said at the annual meeting of the in women’s health care in different states. ly group to be screened for cervical can- mortality from cirrhosis, and violence American Public Health Association. For example, black women have the cer, and they fare worse in cervical cancer against them, Ms. Cohen said. “Racial minorities are projected to make highest rate of Pap smears and the lowest incidence and mortality, she said. This “The Asian American/Pacific Islander up almost half the population by 2050,” rate of osteoporosis, but also have the group has the highest percentage of unin- group fared best in preventive health be- said Ms. -

Making the Capital Connections
9 The Data-Driven Future of Genomics: 5 Minutes with Edico Genome’s CEO LifeLines 11 Navigating a New Policy Year For the California Life Science Community Turning Scientific Discoveries 17 into Successful Companies Making the Capital Connections VOLUME 27, ISSUE 1 Spring 2018 WELCOME IN THIS ISSUE By Joe Panetta, President and CEO, Biocom 4 Cover Story: Making the Capital Connections 9 Guest: The Data-Driven Future of Genomics: 5 Minutes with Edico Genome’s CEO 11 Public Policy: Navigating a Nwq Policy Year Greetings and welcome to our spring 2018 issue of LifeLines! The theme of this issue 13 Biocom Bay Area: Future-Focused and Well Positioned for Continued Success is investment. Investment in our programs and services, and exciting investments in the 15 Biocom LA: Why is Biocom Investing in LA? growth of our association, our members, and in our network of companies, academic 17 Biocom Institute: Turning Scientific Discoveries and research institutes and service providers across the globe. I can proudly declare into Successful Companies that we have now exceeded 1,000 members. In keeping with this growth, our staff 18 Upcoming Biocom Events has expanded to nearly 50 employees and our budget is approaching $10 million 20 Guest: MiraCosta College Launches Inaugural annually. Our footprint now includes members from California, Japan, and across the Biomanufacturing Bachelor's Program EU. This success and our deep bench of talent provide us the power to continue to 21 Biocom Purchasing Group: Investing in expand successfully into markets in the greater Los Angeles area, where we now have Your Success 125 members, and to the north in the San Francisco Bay Area, where we have more 23 San Diego Festival of Science & Engineering: than 200 members.