Pinus Contorta)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yellowstone National Park, Resources and Issues, Vegetation
VEGETATION More than 1,300 plant taxa occur in Yellowstone National Park. The whitebark pine, shown here and found in high elevations in the Greater Yellowstone Ecosystem, is an important native species in decline. Vegetation The vegetation communities of Yellowstone National major disturbances. Yellowstone is home to three Park include overlapping combinations of species endemic plant species, at least two of which depend typical of the Rocky Mountains as well as of the on the unusual habitat created by the park’s thermal Great Plains to the east and the Intermountain region features. Most vegetation management in the park to the west. The exact vegetation community pres- is focused on minimizing human-caused impacts on ent in any area of the park reflects the consequences their native plant communities to the extent feasible. of the underlying geology, ongoing climate change, substrates and soils, and disturbances created by fire, Vegetation Communities floods, landslides, blowdowns, insect infestations, There are several vegetation communities in and the arrival of nonnative plants. Yellowstone: higher- and lower-elevation forests Today, the roughly 1,386 native taxa in the park and the understory vegetation associated with them, represent the species able to either persist in the area sagebrush-steppe, wetlands, and hydrothermal. or recolonize after glaciers, lava flows, and other Quick Facts Number in Yellowstone • Three endemic species (found only Management Issues Native plant taxa: more than 1,300: in Yellowstone): Ross’s bentgrass, • Controlling nonnative species, • Hundreds of wildfowers. Yellowstone sand verbena, which threaten native species, Yellowstone sulfur wild buckwheat. especially near developed areas; • Trees: nine conifers (lodgepole some are spreading into the Nonnative plant species: 225. -

The Introduction of Pinus Contorta in Sweden
The Introduction of Pinus contorta in Sweden Implications for forest diversity Sofia Bäcklund Faculty of Forest Sciences Department of Ecology Uppsala Doctoral Thesis Swedish University of Agricultural Sciences Uppsala 2016 Acta Universitatis Agriculturae Sueciae 2016:30 Cover: A 15-year old Pinus contorta stand. Dorotea, Sweden (photo: S. Bäcklund) ISSN 1652-6880 ISBN (print version) 978-91-576-8562-9 ISBN (electronic version) 978-91-576-8563-6 © 2016 Sofia Bäcklund, Uppsala Print: SLU Service/Repro, Uppsala 2016 The introduction of Pinus contorta in Sweden. Implications for forest diversity Abstract An increasing demand for forest-based products calls for further development and intensification of forest management. The use of non-native tree species in forestry is a common and expanding silvicultural practice worldwide but the effect of non-native trees on native biodiversity and ecosystem functioning is still poorly understood. The general aim of this thesis is to increase our knowledge about what effects large-scale introduction of a non-native tree species have on forest biodiversity over a chronosequence of forest stand ages. The non-native Pinus contorta and the two native tree species Pinus sylvestris and Picea abies were studied over three age classes (15, 30, 85 years old) of managed forests in northern Sweden to compare the stand- and tree structures, the cover and composition of functional groups of ground vegetation, and the species- and functional diversity of epiphytic lichens. Differences in ground vegetation cover were linked to both tree species and different stand and tree characteristics, but the differences were not consistent over the age classes. -
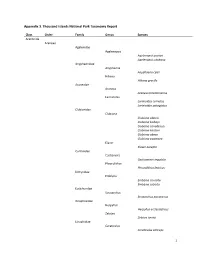
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Protection of Pandora Moth (Coloradia Pandora Blake) Eggs from Consumption by Golden-Mantled Ground Squirrels (Spermophilus Lateralis Say)
AN ABSTRACT OF THE THESIS OF Elizabeth Ann Gerson for the degree of Master of Science in Forest Science presented on 10 January, 1995. Title: Protection of Pandora Moth (Coloradia pandora Blake) Eggs From Consumption by Golden-mantled Ground Squirrels (Spermophilus lateralis Say) Abstract approved: Redacted for Privacy William C. McComb Endemic populations of pandora moths (Coloradia pandora Blake), a defoliator of western pine forests, proliferated to epidemic levels in central Oregon in 1986 and increased dramatically through 1994. Golden-mantled ground squirrels (Spermophilus lateralis Say) consume adult pandora moths, but reject nutritionally valuable eggs from gravid females. Feeding trials with captive S. lateralis were conducted to identify the mode of egg protection. Chemical constituents of fertilized eggs were separated through a polarity gradient of solvent extractions. Consumption of the resulting hexane, dichloromethane, and water egg fractions, and the extracted egg tissue residue, was evaluated by randomized 2-choice feeding tests. Consumption of four physically distinct egg fractions (whole eggs, "whole" egg shells, ground egg shells, and egg contents) also was evaluated. These bioassays indicated that C. pandora eggs are not protected chemically, however, the egg shell does inhibit S. lateralis consumption. Egg protection is one mechanism that enables C. pandora to persist within the forest food web. Spermophilus lateralis, a common and often abundant rodent of central Oregon pine forests, is a natural enemy of C. pandora -

Conifer Reproductive Biology Claire G
Conifer Reproductive Biology Claire G. Williams Conifer Reproductive Biology Claire G. Williams USA ISBN: 978-1-4020-9601-3 e-ISBN: 978-1-4020-9602-0 DOI: 10.1007/978-1-4020-9602-0 Springer Dordrecht Heidelberg London New York Library of Congress Control Number: 2009927085 © Springer Science+Business Media B.V. 2009 No part of this work may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording or otherwise, without written permission from the Publisher, with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Cover Image: Snow and pendant cones on spruce tree (reproduced with permission of Photos.com). Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Foreword When it comes to reproduction, gymnosperms are deeply weird. Cycads and coni- fers have drawn out reproduction: at least 13 genera take over a year from pollina- tion to fertilization. Since they don’t apparently have any selection mechanism by which to discriminate among pollen tubes prior to fertilization, it is natural to won- der why such a delay in reproduction is necessary. Claire Williams’ book celebrates such oddities of conifer reproduction. She has written a book that turns the context of many of these reproductive quirks into deeper questions concerning evolution. The origins of some of these questions can be traced back Wilhelm Hofmeister’s 1851 book, which detailed the revolutionary idea of alternation of generations. -

Connon Nurseries Taylor's Sunburst Lodgepole Pine
Taylor's Sunburst Lodgepole Pine Pinus contorta 'Taylor's Sunburst' Height: 15 feet Spread: 10 feet Sunlight: Hardiness Zone: 4a Description: Wow! When you see this pine tree, you want it - the bright yellow-gold new growth contrasts stunningly with the older green needles for nearly two months in spring, then come the bright red cones; this plant is certain to turn heads in your landscape Ornamental Features Taylor's Sunburst Lodgepole Pine foliage Taylor's Sunburst Lodgepole Pine has attractive green foliage which Photo courtesy of NetPS Plant Finder emerges yellow in spring. The needles are highly ornamental and remain green throughout the winter. The red fruits are held in cones in mid summer. The flowers are not ornamentally significant. The shaggy brick red bark adds an interesting dimension to the landscape. Landscape Attributes Taylor's Sunburst Lodgepole Pine is a multi-stemmed evergreen shrub with an upright spreading habit of growth. Its average texture blends into the landscape, but can be balanced by one or two finer or coarser trees or shrubs for an effective composition. This is a relatively low maintenance shrub. When pruning is necessary, it is recommended to only trim back the new growth of the current season, other than to remove any dieback. It has no significant negative characteristics. Taylor's Sunburst Lodgepole Pine is recommended for the following landscape applications; - Accent - Vertical Accent - General Garden Use Planting & Growing Taylor's Sunburst Lodgepole Pine will grow to be about 15 feet tall at maturity, with a spread of 10 feet. It has a low canopy, and is suitable for planting under power lines. -

Influence of Fire Interval and Serotiny on Postfire Lodgepole Pine Density in Yellowstone National Park
Utah State University DigitalCommons@USU Quinney Natural Resources Research Library, The Bark Beetles, Fuels, and Fire Bibliography S.J. and Jessie E. 2003 Influence of Fire Interval and Serotiny on Postfire Lodgepole Pine Density in Yellowstone National Park Tania Schoennagel Monica G. Turner William H. Romme Follow this and additional works at: https://digitalcommons.usu.edu/barkbeetles Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Forest Biology Commons, Forest Management Commons, and the Wood Science and Pulp, Paper Technology Commons Recommended Citation Schoennagel, T., Turner, M. and Romme, W. (2003). Influence of fire interval and serotiny on postfire lodgepole pine density in Yellowstone National Park. Ecology, 84(11): 2967—2978. This Article is brought to you for free and open access by the Quinney Natural Resources Research Library, S.J. and Jessie E. at DigitalCommons@USU. It has been accepted for inclusion in The Bark Beetles, Fuels, and Fire Bibliography by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Ecology, 84(11), 2003, pp. 2967±2978 q 2003 by the Ecological Society of America THE INFLUENCE OF FIRE INTERVAL AND SEROTINY ON POSTFIRE LODGEPOLE PINE DENSITY IN YELLOWSTONE NATIONAL PARK TANIA SCHOENNAGEL,1,3 MONICA G. TURNER,1 AND WILLIAM H. ROMME2 1Department of Zoology, University of Wisconsin, Madison, Wisconsin 53706 USA 2Department of Forest Sciences, Colorado State University, Fort Collins, Colorado 80523 USA Abstract. The time interval between stand-replacing ®res can in¯uence patterns of initial post®re succession if the abundance of post®re propagules varies with pre®re stand age. -

7. PSEUDOLARIX Gordon, Pinetum 292. 1858, Nom. Cons. 金钱松属 Jin Qian Song Shu Chrysolarix H
Flora of China 4: 41–42. 1999. 7. PSEUDOLARIX Gordon, Pinetum 292. 1858, nom. cons. 金钱松属 jin qian song shu Chrysolarix H. E. Moore; Laricopsis Kent. Trees deciduous; trunk monopodial, straight, terete; branches irregularly whorled; branchlets strongly dimorphic: long branchlets with leaves spirally arranged and radially spreading; short branchlets with leaves radially arranged in false whorls of 10–30 (often spirally spread like a discoid star). Leaves green, turning golden yellow before falling in autumn, narrowly oblanceolate-linear, flattened, 1.5–4 mm wide, flexible, stomatal lines abaxial, in 2 bands, separated by midvein, vascular bundle 1, resin canals 2 or 3 (–7), marginal. Pollen cones terminal on short branchlets, borne in umbellate clusters of 10–25, pendulous at maturity; pollen 2-saccate. Seed cones solitary, shortly pedunculate, erect or ± spreading, ovoid-globose, 2-seeded, maturing in 1st year. Seed scales thick, woody, deciduous at maturity. Bracts adnate to seed scales at base and shed together with them at maturity. Seeds with large, backward projecting wing extending beyond scale margin at maturity. Cotyledons 4–7. 2n = 44*. • One species: China. 1. Pseudolarix amabilis (J. Nelson) Rehder, J. Arnold Arbor. 1: 53. 1919. 金钱松 jin qian song Larix amabilis J. Nelson, Pinaceae 84. 1866; Abies kaempferi Lindley; Chrysolarix amabilis (J. Nelson) H. E. Moore; Laricopsis kaempferi (Lindley) Kent; Pseudolarix fortunei Mayr; P. kaempferi Gordon; P. pourtetii Ferré. Trees to 40 m tall; trunk to 3 m d.b.h.; bark gray-brown, rough, scaly, flaking; crown broadly conical; long branchlets initially reddish brown or reddish yellow, glossy, glabrous, becoming yellowish gray, brownish gray, or rarely purplish brown in 2nd or 3rd year, finally gray or dark gray; short branchlets slow growing, bearing dense rings of leaf cushions; winter buds ovoid, scales free at apex. -

Cedrus Atlantica 'Glauca'
Fact Sheet ST-133 November 1993 Cedrus atlantica ‘Glauca’ Blue Atlas Cedar1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION A handsome evergreen with blue, bluish-green or light green foliage, ‘Glauca’ Atlas Cedar is perfect for specimen planting where it can grow without being crowded since the tree looks its best when branches are left on the tree to the ground (Fig. 1). This shows off the wonderful irregular, open pyramidal form with lower branches spreading about half the height. It grows rapidly when young, then slowly, reaching 40 to 60 feet tall by 30 to 40 feet wide. The trunk stays fairly straight with lateral branches nearly horizontal. Allow plenty of room for these trees to spread. They are best located as a lawn specimen away from walks, streets, and sidewalks so branches will not have to be pruned. It looks odd if lower branches are removed. Older trees become flat-topped and are a beautiful sight to behold. GENERAL INFORMATION Scientific name: Cedrus atlantica ‘Glauca’ Pronunciation: SEE-drus at-LAN-tih-kuh Common name(s): Blue Atlas Cedar Family: Pinaceae USDA hardiness zones: 6 through 8 (Fig. 2) Origin: not native to North America Uses: Bonsai; specimen Availability: generally available in many areas within Figure 1. Young Blue Atlas Cedar. its hardiness range DESCRIPTION Height: 40 to 60 feet Spread: 25 to 40 feet Crown uniformity: irregular outline or silhouette 1. This document is adapted from Fact Sheet ST-133, a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Pinus Contorta Dougl
Unclassified ENV/JM/MONO(2008)32 Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 05-Dec-2008 ___________________________________________________________________________________________ English - Or. English ENVIRONMENT DIRECTORATE JOINT MEETING OF THE CHEMICALS COMMITTEE AND Unclassified ENV/JM/MONO(2008)32 THE WORKING PARTY ON CHEMICALS, PESTICIDES AND BIOTECHNOLOGY Cancels & replaces the same document of 04 December 2008 Series on Harmonisation of Regulatory Oversight in Biotechnology No. 44 CONSENSUS DOCUMENT ON THE BIOLOGY OF LODGEPOLE PINE (Pinus contorta Dougl. ex. Loud.) English - Or. English JT03257048 Document complet disponible sur OLIS dans son format d'origine Complete document available on OLIS in its original format ENV/JM/MONO(2008)32 Also published in the Series on Harmonisation of Regulatory Oversight in Biotechnology: No. 1, Commercialisation of Agricultural Products Derived through Modern Biotechnology: Survey Results (1995) No. 2, Analysis of Information Elements Used in the Assessment of Certain Products of Modern Biotechnology (1995) No. 3, Report of the OECD Workshop on the Commercialisation of Agricultural Products Derived through Modern Biotechnology (1995) No. 4, Industrial Products of Modern Biotechnology Intended for Release to the Environment: The Proceedings of the Fribourg Workshop (1996) No. 5, Consensus Document on General Information concerning the Biosafety of Crop Plants Made Virus Resistant through Coat Protein Gene-Mediated Protection (1996) No. 6, Consensus Document on Information Used in the Assessment of Environmental Applications Involving Pseudomonas (1997) No. 7, Consensus Document on the Biology of Brassica napus L. (Oilseed Rape) (1997) No. 8, Consensus Document on the Biology of Solanum tuberosum subsp. tuberosum (Potato) (1997) No. 9, Consensus Document on the Biology of Triticum aestivum (Bread Wheat) (1999) No. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5.