COSIMA-Rosetta Calibration for In-Situ Characterization of 67P/Churyumov- Gerasimenko Cometary Inorganic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
IOSEA Site Network
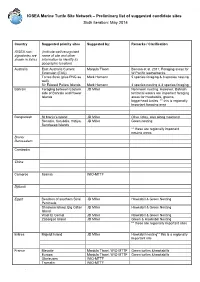
IOSEA Marine Turtle Site Network – Preliminary list of suggested candidate sites Sixth iteration: May 2014 Country Suggested priority sites Suggested by: Remarks / Clarification IOSEA non- (Indicate well-recognized signatories are name of site and other shown in italics information to identify its geographic location) Australia East Australia Current Manjula Tiwari Benson et al. 2011; Foraging areas for Extension (EAC) W.Pacific leatherbacks Torres Strait (plus PNG as Mark Hamann 5 species foraging & 3 species nesting well) Sir Edward Pellew Islands Mark Hamann 3 species nesting & 4 species foraging Bahrain Foraging between Eastern JD Miller No known nesting. However, Bahrain side of Bahrain and Hawar territorial waters are important foraging Islands areas for Hawksbills, greens, loggerhead turtles. ** this is regionally important foraging area Bangladesh St Martin’s Island JD Miller Olive ridley, also along mainland Sonadia, Kutubdia, Hatiya, JD Miller Green nesting Sandweep Islands ** these are regionally important nesting areas Brunei Darussalam Cambodia China Comoros Itsamia WIO-MTTF Djibouti Egypt Beaches of southern Sinai JD Miller Hawksbill & Green Nesting Peninsula Shadwan Island, Big Giftan JD Miller Hawksbill & Green Nesting Island Wadi El Gemal JD Miller Hawksbill & Green Nesting Zabargad Island JD Miller Green & Hawksbill Nesting ** these are regionally important sites Eritrea Mojeidi Island JD Miller Hawksbill nesting** this is a regionally important site France Mayotte Manjula Tiwari, WIO-MTTF Green turtles &hawksbills Europa Manjula Tiwari, WIO-MTTF Green turtles &hawksbills Glorieuses WIO-MTTF Tromelin WIO-MTTF . India Rushikuliya, Odisha (East B.C. Choudhury (ex-WII) Mass nesting ground of Olive Ridley coast of India) K. Sivakumar, Bivash Pandav, K. Suresh Kumar, WII Basudev Tripathy, ZSI Kartik Shanker, IISC and Dakshin Foundation C. -

11973328.Pdf
Foreign Exchange Rate: USD 1 = JPY 96.60 USD 1 = LE 5.56 (Average Betw een March 2009 and August 2009) LOCATIO N MAP (1) LOCATIO N MAP (2) FINALREPORT VOLUME II ENVIRO NMENTALAND SOCIAL IMPACTASSESSMENT O F ABU RAWASH WWTP PROJECT Location Map Table of Contents List of Tables List of Figures Abbreviations Summary TABLEOF CONTENTS CHAPTER 1 INTRODUCTION............................................................................................1-1 1.1 Background.....................................................................................................1-1 1.2 Objective ofthe Environmental and Social Impact Assessment Study..................1-2 1.3 Report Structure...............................................................................................1-3 CHAPTER 2 LEGAL AND ADMINISTRATIVE FRAMEWORK..........................................2-1 2.1 Administrative Framework................................................................................2-1 2.1.1 Construction Authority for Potable Water andWastewater............................2-2 2.1.2 Holding Company for Water andWastewater..............................................2-3 2.1.3 Cairo Sanitary Drainage Company..............................................................2-4 2.1.4 Cairo Water Supply Company....................................................................2-4 2.1.5 Giza Water and Wastewater Company.........................................................2-5 2.1.6 Egyptian Environmental AffairsAgency.....................................................2-5 -

Ancient Stone Quarry Landscapes In
QuarryScapes: quarry stone ancient Mediterranean landscapes in the Eastern QuarryScapes: ancient QuarryScapes:stone quarry landscapes ancient stone in quarrythe Eastern landscapes Mediterranean in the EasternGeological Survey of MediterraneanNorway, Special Publication, 12 Geological Survey of Norway, Special Publication, 12 Geological Survey of Norway, Special Publication, 12 Abu-Jaber et al. (eds.) et al. 12 Abu-Jaber Special Publication, Geological Survey of Norway, Abu-Jaber, N., Bloxam, E.G., Degryse,P. and Heldal, T. (eds.) Geological Survey of Norway, Special Publication, 12 The NGU Special Publication series comprises consecutively numbered volumes containing papers and proceedings from national and international symposia or meetings dealing with Norwegian and international geology, geophysics and geochemistry; excursion guides from such symposia; and in some cases papers of particular value to the international geosciences community, or collections of thematic articles. The language of the Special Publication series is English. Editor: Trond Slagstad ©2009 Norges geologiske undersøkelse Published by Norges geologiske undersøkelse (Geological Survey of Norway) NO-7491 Norway All Rights reserved ISSN: 0801-5961 ISBN: 978-82-7385-138-3 Design and print: Trykkpartner Grytting AS Cover illustration: Situated far out in the Eastern Desert in Egypt, Mons Claudianus is one of the most spectacular quarry landscapes in Egypt. The white tonalite gneiss was called marmor claudianum by the Romans, and in particular it was used for large objects such as columns and bathtubs. Giant columns of the stone can be seen in front of Pantheon in Rome. Photo by Tom Heldal. GEOLOGICAL SURVEY OF NORWAY SPECIAL PUBLICATION n Contents Introduction Abu-Jaber, N., Bloxam, E.G., Degryse, P. -

Chicago, Illinois
The 62nd Annual Meeting of the American Research Center in Egypt April 1-3, 2011 Chicago Marriott Downtown Chicago, Illinois Cairo Midan Simon Bolivar February 2011 PAPERS ON ARCHAEOLOGY OF THE LEIDEN MUSEUM OF ANTIQUITIES Cary J. Martin Demotic Papyri from the Memphite Necropolis In the Collections of the National Museum of Antiquities in Leiden, the British Museum and the Hermitage Museum 2 vols., 263 p., 66 b/w ills., 220 x 280 mm, 2009, PALMA 5, PB, ISBN 978-2-503-53353-7, $115.00 e Demotic texts published in this volume come from the Archives of the funerary-workers of the Memphite Necropolis. Full photographs of each papyrus are provided and detailed indexes complete the publication. Maarten J. Raven, Vincent Verschoor, Marije Vugts, René van Walsem e Memphite Tomb of Horemheb Commander-in-Chief of Tutankhamun,V: e Forecourt and the Area South of the Tomb with Some Notes on the Tomb of Tia 403 p., 220 x 280 mm, 2011, PALMA 6, PB, ISBN 978-2-503-53110-6, $123.00 is book is the rst in a series dealing with the excavations in the New Kingdom cemetery of Saqqara. e tomb of the general Horemheb is the most important monument of this cemetery. Please add 6% CT Sales Tax, $5.00 shipping for the rst book, and $2.50 for each add’l [email protected] • www.brepols.net DAVID BROWN BOOK COMPANY (orders North America) PO Box 511 (28 Main Street) • Oakville, CT 06779 (USA) T: 800 791 9354 (toll-free); 860 945 9329 • F: 860 945 9468 [email protected] • www.oxbowbooks.com ARCE Chapter Council 2011 Fundraiser in support of the EgyptBest Student in Chicago!Paper Contest The Legacy of Ancient Egypt in Chicago’s Architecture by Michael Berger Saturday, April 2, 2011 12:15pm – 1:00pm Clark, 4th Floor, Marriott Hotel $15.00 per person, tickets must be purchased in advance Museums, Monuments, and Archives: Fellowship Opportunities with ARCE Saturday April 2, 4:15 - 5:15pm Belmont Room, 4th Floor Join former ARCE fellows and the ARCE Academic Coordinator to hear first hand accounts about conducting research in Egypt as pre and post doctoral scholars. -

Zabargad: the Ancient Peridot Island in the Red Sea
ZABARGAD: THE ANCIENT PERIDOT ISLAND IN THE RED SEA By Edward Giibelin Reflecting on his 1980 visit to Zabargad, abargad is the oldest and longest-lznown source the author provides 11ti update on this zof gem peridots (fig. 1); yet the island, just as relatively unknown island and the much as the gemstone it hoarded, has slid repeatedly into bcarrtiful peridots for which it has gained oblivion, only to be rediscovered over and over again and fame. He con~plementshis discussion of forgotten once more. The author visited this tiny island the geology of Zal?agad, the peridot in the Red Sea in March of 1980. Located about 60 miles occurrences, the mining techniques, and southeast of the RBs Bands peninsula, at 23" 36' 16" N the characteristics of the gemstone with a look ut the al~cienthistory of the island and 36" 11' 42" E (fig. 2))it is situated 16 lzm north of the and the etymological cliangcs Tropic of Cancer. Zabargad is only 3.2 lzm long and 2.4 surrounding the stone's current name, lzm wide, covering an area of 4.5 lzm2. There is hardly peridot. Although somewhat low on the any life on the island and no fresh water at all; one may hardness scale, this n~agnlficentgemstone justly describe it as a "desert island." In fact, apart from has regained popufrrr appreciation, and the low-growing shrubs, several giant turtles, and a few birds crystals from Zabargud are still arnolig the such as wagtails, ospreys, and gulls, practically no flora finest in the world. -

The Eastern Desert in Late Antiquity Jean-Luc Fournet
The Eastern Desert in Late Antiquity Jean-Luc Fournet To cite this version: Jean-Luc Fournet. The Eastern Desert in Late Antiquity. Jean-Pierre Brun. The Eastern Desert of Egypt during the Greco-Roman Period: Archaeological Reports, Collège de France, 2018, 10.4000/books.cdf.5242. hal-01991148 HAL Id: hal-01991148 https://hal.archives-ouvertes.fr/hal-01991148 Submitted on 23 Jan 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Jean-Pierre Brun, Thomas Faucher, Bérangère Redon and Steven Sidebotham (dir.) The Eastern Desert of Egypt during the Greco-Roman Period: Archaeological Reports Collège de France The Eastern Desert in Late Antiquity Jean-Luc Fournet DOI: 10.4000/books.cdf.5242 Publisher: Collège de France Place of publication: Paris Year of publication: 2018 Published on OpenEdition Books: 14 September 2018 Serie: Institut des civilisations Electronic ISBN: 9782722604889 http://books.openedition.org Brought to you by Collège de France Electronic reference FOURNET, Jean-Luc. The Eastern Desert in Late Antiquity In: The Eastern Desert of Egypt during the Greco- Roman Period: Archaeological Reports [online]. Paris: Collège de France, 2018 (generated 22 octobre 2018). Available on the Internet: <http://books.openedition.org/cdf/5242>. -

Hydrogen Incorporation in Natural Mantle Olivines
Hydrogen Incorporation in Natural Mantle Olivines Jed L. Mosenfelder1, Thomas G. Sharp2, 1 Paul D. Asimow , and George R. Rossman' Constraints on water storage capacity and actual content in the mantle must be derived not only from experimental studies, but also from investigation of natural samples. Olivine is one of the best-studied, OH-bearing "nominally anhydrous" minerals, yet there remain multiple hypotheses for the incorporation mechanism of hydrogen in this phase. Moreover, there is still debate as to whether the mechanism is the same in natural samples vs. experimental studies, where concentrations can reach very high values (up to ~o.6 wt% H20) at high pressures and temperatures. We present new observations and review IR and TEM data from the literature that bear on this question. Hydrogen incorporation in natural olivine clearly occurs by multiple mechanisms, but in contrast to some previous assertions we find that there are strong similarities between the IR signatures of experimentally annealed olivines and most natural samples. At low pressures (lower than ~2 GPa) in both experiments and natural olivines, hydrogen incorporation might be dominated by a humite-type defect, but the nature of the defect may vary even within a single sample; possibilities include point defects, planar defects and optically detectable 1 inclusions. IR bands between 3300 and 3400 cm- , ascribed previously to the influ ence of silica activity, are apparently related instead to increased oxygen fugacity. At higher pressures in experiments, the IR band structure changes and hydrogen is probably associated with disordered point defects. Similar IR spectra are seen in olivines from xenoliths derived from deeper parts of the mantle (below South Africa and the Colorado Plateau) as well as in olivines from the ultra-high pressure metamorphic province of the Western Gneiss Region in Norway. -

Hydrogen in Olivines from the Colorado Plateau: Implications for Water in the Mantle and the Alpe Arami Controversy
Hydrogen in Olivines From the Colorado Plateau: Implications for Water in the Mantle and the Alpe Arami Controversy Identifier T32B-07 Mosenfelder, J L*, GPS Caltech, M/C 170-25, Pasadena, CA 91125 United States Sharp, T G, Dept of Geol Sci, ASU, Box 871404, Tempe, AZ 85287 United States Authors Asimow, P, GPS Caltech, M/C 170-25, Pasadena, CA 91125 United States Rossman, G R, GPS Caltech, M/C 170-25, Pasadena, CA 91125 United States Abstract Petrological, geochemical and geophysical data indicate that portions of the upper mantle beneath the Colorado Plateau are highly hydrated, which has important implications for mantle viscosity and geodynamics (e.g. [1]). One line of evidence for this comes from the study of H in nominally anhydrous minerals. It has been shown that mantle garnets from the Plateau have higher H contents than those from other continental areas. Recently, we began a reinvestigation of H in olivines from the Buell Park (Arizona) and Green Knobs (New Mexico) diatremes, using polarized IR spectroscopy. Two varieties of olivine have been described [2] at these localities: green-colored crystals containing trace amounts of H, and brown olivines that contain substantially more H in the form of lamellae of Ti-clinohumite (Ti-cl). Our new observations show that there is a continuum between these two varieties. H concentrations range from 50 to 530 wt ppm H2O. The olivine with the lowest H content exhibits strong absorption bands in the 3400 to 3650 cm-1 wavenumber region that closely resemble (in location and pleochroism) those found in olivines hydrated in our laboratory and by Kohlstedt and co-workers. -

556 INDE X INDE X 1973 War 43 a Abdeen Palace 117 Abu Ghorab
© Lonely Planet Publications 556 Index (B-C) 557 bargaining 514 bus travel to/from Egypt 524-5 Cairo International Film Festival 510 INDEX Baring, Sir Evelyn 40 bus travel within Egypt 529, 532 Cairo Tower 142 Baris 336 business hours 503 Cairo Zoo 156-7 INDEX Index Bashendi 342 Cambyses 346 Bastet 52 C camel markets 1973 War 43 attractions 373-85 rock art 298 bathrooms 516 Cairo 107-84, 110-11 Birqash 212-13 beaches 384-5 rock carvings 341 Battle of the Pyramids 38 accommodation 157-62 Daraw 299 A Central Alexandria 378-9, art galleries Bawiti 349-53, 350 activities 153-5 camel rides Abdeen Palace 117 380-1 Cairo 153 beaches Agouza 142, 144-5 Dakhla Oases 341 Abu Ghorab 199 courses 385 Gezira Art Center 141 Alexandria 384-5 ahwas 169-70 Giza Plateau 147 Abu Giffa Pass 493 drinking 389-91 Hanager Arts Centre 141 Hurghada 424 attractions 116-53 Luxor 276 Abu Mina 395 Eastern Suburbs 381-4 Palace of Arts 141 Ismailia 413 bookshops 109-12 Sharm el-Sheikh 466 Abu Qir 394-5 entertainment 391-2 arts 69-76 Mediterranean Coast 403 Central Cairo 116-23, 118-19 camel safaris Abu Simbel 22, 323-6, 12 food 387-9 Aswan 83-4, 299-315, 302, 9 Moon Beach 459 children, travel with 156-7 Nuweiba 481-3 Abu Sir 196-9, 199 history 369-70 accommodation 309-12 Ras Mohammed National Park cinemas 171-3 Sinai 475 Abydos 233-4, 233 internet access 371 activities 308-9 461-2 Coptic Cairo 123-5 camping 500 accommodation 19, 158, 500-2, see itineraries 24, 28, 371 attractions 301-8 Bedouin people 65 cultural centres 112-13 Canyon, the 445, 475 also individual locations -

EGU2014-13367, 2014 EGU General Assembly 2014 © Author(S) 2014
Geophysical Research Abstracts Vol. 16, EGU2014-13367, 2014 EGU General Assembly 2014 © Author(s) 2014. CC Attribution 3.0 License. Petrology of pyroxenitic vein in spinel-plagioclas lherzolites from Zabargad island, Red Sea, Egypt Ingrid Gjerazi and Theodoros Ntaflos Dept. of Lithospheric Research, University of Vienna, Vienna, Austria ([email protected]) The island of Zabargad (St. John’s island), located off the coast of Egypt contains three peridotite bodies, which comprise of Spinel- and Plagioclase-Lherzolithes. The studied rock is a composite peridotite consisting of a pyroxenitic vein with coarse grained augites of approx. 3 cm, originating from the Main Peridotite Hill (MPH) in Zabargad. Information on the nature and formation of this peridotite from Zabargad was obtained through the miner- alogical and petrographic analysis, using a polarizing light microscope and electron microprobe, of five thin sections originating from the aforementioned peridotite. Furthermore, the chemical heterogeneities and the modal changes of the peridotite through its interaction with intrusive pyroxenitic veins were studied in order to create a better understanding of the origin of the rock and of the geology of the island. Results have shown, that the mineralogical composition of this peridotite comprises of: olivine, clinopyrox- ene, orthopyroxene, plagioclase, amphibole and spinel. In the host rock, the Fo (forsterite) content of olivine varies from approx. 88% to 89% and in the vein it remains well within the boundaries of 89%. Compared to primary xenoliths originating from the fertile, undepleted mantle with an Mg-number (100*Mg#), which ranges from 89.2% to 89.9%, the similarities with the olivines can clearly be seen. -

560 INDEX a Ababda People 438 Aboukir 393 Abu
© Lonely Planet 560 Index A eastern suburbs 380-2 nautical archaeology 376 Ababda people 438 entertainment 390 recent finds 204, 261, 297, 376, Aboukir 393 food 386-8 399 Abu 304-5 history 369-70 architecture 76 Abu Ghorab 197-200 internet access 371 books 129 Abu Simbel 323-5, 12 itineraries 23, 29, 371, 388, 23, 29 Ottoman architecture 134-5, Abu Sir 194-8, 197 markets 382-3 341-2, 394-5, 434 Abydos 233-5, 233 medical services 372 tombs 54-5, 259 accommodation 21, 504-6, see shopping 390-1 art, see also art galleries, arts also individual destinations tourist information 373-4 Coptic Christianity 420 language 543 tours 383 festivals 512, 513 activities 506-7, see also individual travel to/from 391-2 hieroglyphs 60-1 activities travel within 392-3 Pharaonic Egypt 56-9, 200, 208 Ad-Dahar 426-8, 430, 427 Alexandria National Museum 375-6 rock carvings 340, 438, 464 Aghurmi 358 Al-Faraun 203 art galleries, see also museums Agilkia Island 315-16, 316 Al-Fayoum Oasis 205-11 Cairo Atelier 155 ahwas 81 Al-Gedida 342 Contemporary Image Collective Alexandria 388-9 al-Ghitani, Gamal 72 155 Cairo 170 al-Ghouri, Sultan 39 Coptic Museum 126 Ain al-Furtega 487 Al-Kab 292-3 Gezira Art Centre 142 Ain Della 346 al-Khamissi, Khalid 73 Hanager Arts Centre 143 Ain Musa 462-3 Al-Kharga 329-34, 332 Karim Francis Art Gallery 155 INDEX Ain Safi 359 Al-Kharga Oasis 329-35, 330 Mahmoud Mukhtar Museum 143 Ain Umm el-Dabadib 335 Al-Qasr 341-2 Mahmoud Said Museum 381 air travel Al-Quseir 433-5, 452, 456, 434 Mashrabia Gallery of Contempo- to/from Egypt 524-5 Al-Qusiya -

Redseaturtles.Pdf
Biological Conservation 261 (2021) 109235 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Distribution and temporal trends in the abundance of nesting sea turtles in the Red Sea Takahiro Shimada a,b,c,d,*, Mark G. Meekan a, Robert Baldwin e, Abdulaziz M. Al-Suwailem f, Christopher Clarke f, August S. Santillan f, Carlos M. Duarte b a Australian Institute of Marine Science, Crawley, Western Australia 6009, Australia b Red Sea Research Center and Computational Bioscience Research Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia c UWA Oceans Institute and School of Biological Sciences, University of Western Australia, Crawley, WA, Australia d Department of Environment and Science, Queensland Government, GPO Box 2454, Dutton Park, QLD 4001, Australia e Five Oceans (Environmental Services) LLC, Box 660, PC 131, Oman f Beacon Development Company, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia ARTICLE INFO ABSTRACT Keywords: Mobile species often aggregate at predictable places and times to ensure that individuals findmates and breed in Population ecology suitable habitats. Sea turtles demonstrate this life history trait, which can make these species highly susceptible Coastal development to population declines if nesting habitats are lost or degraded. Conservation management thus requires knowl Climate change edge of where and when turtles nest and changes in abundance in these habitats through time. Here, we Red Sea compiled new and published data and used a novel analysis to describe seasonality, annual abundance and Nesting seasonality Sea turtle spatial distribution of nesting green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles in data- deficient populations that inhabit the Red Sea.