Differential Interactions Between Notch and ID Factors Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RBP-J Signaling − Cells Through Notch Novel IRF8-Controlled
Sca-1+Lin−CD117− Mesenchymal Stem/Stromal Cells Induce the Generation of Novel IRF8-Controlled Regulatory Dendritic Cells through Notch −RBP-J Signaling This information is current as of September 25, 2021. Xingxia Liu, Shaoda Ren, Chaozhuo Ge, Kai Cheng, Martin Zenke, Armand Keating and Robert C. H. Zhao J Immunol 2015; 194:4298-4308; Prepublished online 30 March 2015; doi: 10.4049/jimmunol.1402641 Downloaded from http://www.jimmunol.org/content/194/9/4298 Supplementary http://www.jimmunol.org/content/suppl/2015/03/28/jimmunol.140264 http://www.jimmunol.org/ Material 1.DCSupplemental References This article cites 59 articles, 19 of which you can access for free at: http://www.jimmunol.org/content/194/9/4298.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 25, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2015 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Sca-1+Lin2CD1172 Mesenchymal Stem/Stromal Cells Induce the Generation of Novel IRF8-Controlled Regulatory Dendritic Cells through Notch–RBP-J Signaling Xingxia Liu,*,1 Shaoda Ren,*,1 Chaozhuo Ge,* Kai Cheng,* Martin Zenke,† Armand Keating,‡,x and Robert C. -

Single-Cell RNA-Sequencing-Based Crispri Screening Resolves Molecular Drivers of Early Human Endoderm Development
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2019-04-16 Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Ryan M. Genga University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Cell Biology Commons, Cells Commons, Developmental Biology Commons, Embryonic Structures Commons, Genetic Phenomena Commons, and the Nucleic Acids, Nucleotides, and Nucleosides Commons Repository Citation Genga RM, Kernfeld EM, Parsi KM, Parsons TJ, Ziller MJ, Maehr R. (2019). Single-Cell RNA-Sequencing- Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development. Open Access Articles. https://doi.org/10.1016/j.celrep.2019.03.076. Retrieved from https://escholarship.umassmed.edu/oapubs/3818 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Report Single-Cell RNA-Sequencing-Based CRISPRi Screening Resolves Molecular Drivers of Early Human Endoderm Development Graphical Abstract Authors Ryan M.J. Genga, Eric M. Kernfeld, Krishna M. Parsi, Teagan J. Parsons, Michael J. Ziller, Rene´ Maehr Correspondence [email protected] In Brief Genga et al. utilize a single-cell RNA- sequencing-based CRISPR interference approach to screen transcription factors predicted to have a role in human definitive endoderm differentiation. -

Mutations and Altered Expression of SERPINF1 in Patients with Familial Otosclerosis Joanna L
Human Molecular Genetics, 2016, Vol. 25, No. 12 2393–2403 doi: 10.1093/hmg/ddw106 Advance Access Publication Date: 7 April 2016 Original Article ORIGINAL ARTICLE Mutations and altered expression of SERPINF1 in patients with familial otosclerosis Joanna L. Ziff1, Michael Crompton1, Harry R.F. Powell2, Jeremy A. Lavy2, Christopher P. Aldren3, Karen P. Steel4,†, Shakeel R. Saeed1,2 and Sally J. Dawson1,* 1UCL Ear Institute, University College London, London WC1X 8EE, UK, 2Royal National Throat Nose and Ear Hospital, London WC1X 8EE, UK, 3Department of ENT Surgery, The Princess Margaret Hospital, Windsor SL4 3SJ, UK and 4Wellcome Trust Sanger Institute, Hinxton CB10 1SA, UK *To whom correspondence should be addressed. Tel: þ44 2076798935; Email: [email protected] Abstract Otosclerosis is a relatively common heterogenous condition, characterized by abnormal bone remodelling in the otic capsule leading to fixation of the stapedial footplate and an associated conductive hearing loss. Although familial linkage and candidate gene association studies have been performed in recent years, little progress has been made in identifying disease- causing genes. Here, we used whole-exome sequencing in four families exhibiting dominantly inherited otosclerosis to identify 23 candidate variants (reduced to 9 after segregation analysis) for further investigation in a secondary cohort of 84 familial cases. Multiple mutations were found in the SERPINF1 (Serpin Peptidase Inhibitor, Clade F) gene which encodes PEDF (pigment epithelium-derived factor), a potent inhibitor of angiogenesis and known regulator of bone density. Six rare heterozygous SERPINF1 variants were found in seven patients in our familial otosclerosis cohort; three are missense mutations predicted to be deleterious to protein function. -

Hes5 Regulates the Transition Timing of Neurogenesis and Gliogenesis In
© 2017. Published by The Company of Biologists Ltd | Development (2017) 144, 3156-3167 doi:10.1242/dev.147256 RESEARCH ARTICLE Hes5 regulates the transition timing of neurogenesis and gliogenesis in mammalian neocortical development Shama Bansod1,2, Ryoichiro Kageyama1,2,3,4 and Toshiyuki Ohtsuka1,2,3,* ABSTRACT has been reported that the high mobility group AT-hook (Hgma) During mammalian neocortical development, neural stem/progenitor genes regulate gene expression by modulating chromatin structure cells (NSCs) sequentially give rise to deep layer neurons and (Ozturk et al., 2014), maintain neurogenic NSCs, and inhibit superficial layer neurons through mid- to late-embryonic stages, gliogenesis during early- to mid-embryonic stages through global shifting to gliogenic phase at perinatal stages. Previously, we found opening of the chromatin state (Kishi et al., 2012). However, the that the Hes genes inhibit neuronal differentiation and maintain mechanism by which the expression of these epigenetic factors is NSCs. Here, we generated transgenic mice that overexpress Hes5 in controlled remains to be analyzed. NSCs of the central nervous system, and found that the transition Here, we found that Hes5, a transcriptional repressor acting timing from deep to superficial layer neurogenesis was shifted earlier, as an effector of Notch signaling, regulates the timing of while gliogenesis precociously occurred in the developing neocortex neurogenesis and gliogenesis via alteration in the expression of Hes5-overexpressing mice. By contrast, the transition from deep to levels of epigenetic factors. Notch signaling contributes to the superficial layer neurogenesis and the onset of gliogenesis were elaboration of cellular diversity during the development of various delayed in Hes5 knockout (KO) mice. -

Single Cell Transcriptomics Reveal Temporal Dynamics of Critical Regulators of Germ Cell Fate During Mouse Sex Determination
bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Single cell transcriptomics reveal temporal dynamics of critical regulators of germ 2 cell fate during mouse sex determination 3 Authors: Chloé Mayère1,2, Yasmine Neirijnck1,3, Pauline Sararols1, Chris M Rands1, 4 Isabelle Stévant1,2, Françoise Kühne1, Anne-Amandine Chassot3, Marie-Christine 5 Chaboissier3, Emmanouil T. Dermitzakis1,2, Serge Nef1,2,*. 6 Affiliations: 7 1Department of Genetic Medicine and Development, University of Geneva, 1211 Geneva, 8 Switzerland; 9 2iGE3, Institute of Genetics and Genomics of Geneva, University of Geneva, 1211 10 Geneva, Switzerland; 11 3Université Côte d'Azur, CNRS, Inserm, iBV, France; 12 Lead Contact: 13 *Corresponding Author: Serge Nef, 1 rue Michel-Servet CH-1211 Genève 4, 14 [email protected]. + 41 (0)22 379 51 93 15 Running Title: Single cell transcriptomics of germ cells 1 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 16 Abbreviations; 17 AGC: Adrenal Germ Cell 18 GC: Germ cell 19 OGC: Ovarian Germ Cell 20 TGC: Testicular Germ Cell 21 scRNA-seq: Single-cell RNA-Sequencing 22 DEG: Differentially Expressed Gene 23 24 25 Keywords: 26 Single-cell RNA-Sequencing (scRNA-seq), sex determination, ovary, testis, gonocytes, 27 oocytes, prospermatogonia, meiosis, gene regulatory network, germ cells, development, 28 RNA splicing 29 2 bioRxiv preprint doi: https://doi.org/10.1101/747279; this version posted November 2, 2020. -

Olig2 and Ngn2 Function in Opposition to Modulate Gene Expression in Motor Neuron Progenitor Cells
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells Soo-Kyung Lee,1 Bora Lee,1 Esmeralda C. Ruiz, and Samuel L. Pfaff2 Gene Expression Laboratory, The Salk Institute for Biological Studies, La Jolla, California 92037, USA Spinal motor neurons and oligodendrocytes are generated sequentially from a common pool of progenitors termed pMN cells. Olig2 is a bHLH-class transcription factor in pMN cells, but it has remained unclear how its transcriptional activity is modulated to first produce motor neurons and then oligodendrocytes. Previous studies have shown that Olig2 primes pMN cells to become motor neurons by triggering the expression of Ngn2 and Lhx3. Here we show that Olig2 also antagonizes the premature expression of post-mitotic motor neuron genes in pMN cells. This blockade is counteracted by Ngn2, which accumulates heterogeneously in pMN cells, thereby releasing a subset of the progenitors to differentiate and activate expression of post-mitotic motor neuron genes. The antagonistic relationship between Ngn2 and Olig2 is mediated by protein interactions that squelch activity as well as competition for shared DNA-binding sites. Our data support a model in which the Olig2/Ngn2 ratio in progenitor cells serves as a gate for timing proper gene expression during the development of pMN cells: Olig2high maintains the pMN state, thereby holding cells in reserve for oligodendrocyte generation, whereas Ngn2high favors the conversion of pMN cells into post-mitotic motor neurons. [Keywords: Motor neuron; oligodendrocyte; development; basic helix–loop–helix (bHLH); neurogenin (Ngn); Olig] Supplemental material is available at http://www.genesdev.org. -

Watsonjn2018.Pdf (1.780Mb)
UNIVERSITY OF CENTRAL OKLAHOMA Edmond, Oklahoma Department of Biology Investigating Differential Gene Expression in vivo of Cardiac Birth Defects in an Avian Model of Maternal Phenylketonuria A THESIS SUBMITTED TO THE GRADUATE FACULTY In partial fulfillment of the requirements For the degree of MASTER OF SCIENCE IN BIOLOGY By Jamie N. Watson Edmond, OK June 5, 2018 J. Watson/Dr. Nikki Seagraves ii J. Watson/Dr. Nikki Seagraves Acknowledgements It is difficult to articulate the amount of gratitude I have for the support and encouragement I have received throughout my master’s thesis. Many people have added value and support to my life during this time. I am thankful for the education, experience, and friendships I have gained at the University of Central Oklahoma. First, I would like to thank Dr. Nikki Seagraves for her mentorship and friendship. I lucked out when I met her. I have enjoyed working on this project and I am very thankful for her support. I would like thank Thomas Crane for his support and patience throughout my master’s degree. I would like to thank Dr. Shannon Conley for her continued mentorship and support. I would like to thank Liz Bullen and Dr. Eric Howard for their training and help on this project. I would like to thank Kristy Meyer for her friendship and help throughout graduate school. I would like to thank my committee members Dr. Robert Brennan and Dr. Lilian Chooback for their advisement on this project. Also, I would like to thank the biology faculty and staff. I would like to thank the Seagraves lab members: Jailene Canales, Kayley Pate, Mckayla Muse, Grace Thetford, Kody Harvey, Jordan Guffey, and Kayle Patatanian for their hard work and support. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -
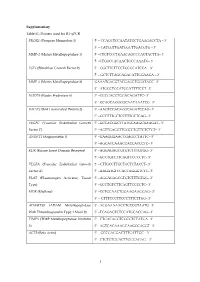
1 Supplementary Table S1. Primers Used for RT-Qpcr PROX1
Supplementary Table S1. Primers used for RT-qPCR PROX1 (Prospero Homeobox 1) 5’ – CCAGCTCCAATATGCTGAAGACCTA – 3’ 5’ – CATCGTTGATGGCTTGACGTG – 3‘ MMP-1 (Matrix Metallopeptidase 1) 5' –CTGTCCCTGAACAGCCCAGTACTTA– 3' 5' –CTGGCCACAACTGCCAAATG– 3' FGF2 (Fibroblast Growth Factor 2) 5′ - GGCTTCTTCCTGCGCATCCA – 3′ 5′ – GCTCTTAGCAGACATTGGAAGA – 3′ MMP-3 (Matrix Metallopeptidase 3) GAAATGAGGTACGAGCTGGATACC– 3’ 5’ –ATGGCTGCATCGATTTTCCT– 3’ NUDT6 (Nudix Hydrolase 6) 5’ –GGCGAGCTGGACAGATTC– 3’ 5’ –GCAGCAGGGGCAATAAATCG– 3’ BAIAP2 (BAI1 Associated Protein 2) 5’ –AAGTCCACAGGCAGATCCAG– 3’ 5’ –GCCTTTGCTCCTTTGCTCAG– 3’ VEGFC (Vascular Endothelial Growth 5’ –GCCACGGCTTATGCAAGCAAAGAT– 3’ Factor C) 5’ –AGTTGAGGTTGGCCTGTTCTCTGT– 3’ ANGPT1 (Angiopoietin 1) 5’ –GAAGGGAACCGAGCCTATTC– 3’ 5’ –AGCATCAAACCACCATCCTC– 3’ KDR (Kinase Insert Domain Receptor) 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ VEGFA (Vascular Endothelial Growth 5’ –CTTGCCTTGCTGCTCTACCT– 3’ Factor A) 5’ –AAGATGTCCACCAGGGTCTC– 3’ PLAT (Plasminogen Activator, Tissue 5’ –AGGAGAGCGTGTCTTTGTGG– 3’ Type) 5’ –GCCTGTCTTCAGTTCCCCTC– 3’ MDK (Midkine) 5’ –CCTGCAACTGGAAGAAGGAG– 3’ 5’ -- CTTTCCCTTCCCTTTCTTGG– 3’ ADAMTS9 (ADAM Metallopeptidase 5’ –ACGAAAAACCTGCCGTAATG– 3’ With Thrombospondin Type 1 Motif 9) 5’ –TCAGAGTCTCCATGCACCAG– 3’ TIMP3 (TIMP Metallopeptidase Inhibitor 5’ –CTGACAGGTCGCGTCTATGA– 3’ 3) 5’ –AGTCACAAAGCAAGGCAGGT– 3’ ACTB (Beta Actin) 5’ – GCCGAGGACTTTGATTGC – 3’ 5’– CTGTGTGGACTTGGGAGAG – 3’ 1 Figure S1. Efficient silencing of PROX1 in CGTH-W-1 and FTC-133 cells. Western blotting analysis shows a decrease in PROX1 protein level by targeting with siRNAs purchased from Santa Cruz (SC) and Sigma-Aldrich (SA) in both CGTH-W-1 and FTC-133 cell line. Beta-actin was used as a loading control of protein lysates. Figure S2. The tube formation assay. The silencing of PROX1 in CGTH-W-1 and FTC-133 cells enhances the angiogenesis in vitro of endothelial cells. HUVECs were cultured in 96-well plates coated with a semi-solid Matrigel. -

Landscape of Transcriptional Deregulation in Lung Cancer Shu Zhang1,2,3,4, Mingfa Li1, Hongbin Ji2,3,4,5* and Zhaoyuan Fang2,3,4,6*
Zhang et al. BMC Genomics (2018) 19:435 https://doi.org/10.1186/s12864-018-4828-1 RESEARCHARTICLE Open Access Landscape of transcriptional deregulation in lung cancer Shu Zhang1,2,3,4, Mingfa Li1, Hongbin Ji2,3,4,5* and Zhaoyuan Fang2,3,4,6* Abstract Background: Lung cancer is a very heterogeneous disease that can be pathologically classified into different subtypes including small-cell lung carcinoma (SCLC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC) and large-cell carcinoma (LCC). Although much progress has been made towards the oncogenic mechanism of each subtype, transcriptional circuits mediating the upstream signaling pathways and downstream functional consequences remain to be systematically studied. Results: Here we trained a one-class support vector machine (OC-SVM) model to establish a general transcription factor (TF) regulatory network containing 325 TFs and 18724 target genes. We then applied this network to lung cancer subtypes and identified those deregulated TFs and downstream targets. We found that the TP63/SOX2/ DMRT3 module was specific to LUSC, corresponding to squamous epithelial differentiation and/or survival. Moreover, the LEF1/MSC module was specifically activated in LUAD and likely to confer epithelial-to-mesenchymal transition, known important for cancer malignant progression and metastasis. The proneural factor, ASCL1, was specifically up-regulated in SCLC which is known to have a neuroendocrine phenotype. Also, ID2 was differentially regulated between SCLC and LUSC, with its up-regulation in SCLC linking to energy supply for fast mitosis and its down-regulation in LUSC linking to the attenuation of immune response. We further described the landscape of TF regulation among the three major subtypes of lung cancer, highlighting their functional commonalities and specificities. -

Modeling the Phenotype of Spinal Muscular Atrophy by the Direct Conversion of Human Fibroblasts to Motor Neurons
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 7), pp: 10945-10953 Research Paper Modeling the phenotype of spinal muscular atrophy by the direct conversion of human fibroblasts to motor neurons Qi-Jie Zhang1,*, Jin-Jing Li1,*, Xiang Lin1, Ying-Qian Lu1, Xin-Xin Guo1, En-Lin Dong1, Miao Zhao1, Jin He1, Ning Wang1,2 and Wan-Jin Chen1,2 1 Department of Neurology and Institute of Neurology, First Affiliated Hospital, Fujian Medical University, Fuzhou, China 2 Fujian Key Laboratory of Molecular Neurology, Fuzhou, China * These authors have contributed equally to this work Correspondence to: Wan-Jin Chen, email: [email protected] Keywords: direct reprogramming; fibroblast; induced motor neuron; spinal muscular atrophy Received: June 08, 2016 Accepted: November 22, 2016 Published: January 13, 2017 ABSTRACT Spinal muscular atrophy (SMA) is a lethal autosomal recessive neurological disease characterized by selective degeneration of motor neurons in the spinal cord. In recent years, the development of cellular reprogramming technology has provided an alternative and effective method for obtaining patient-specific neuronsin vitro. In the present study, we applied this technology to the field of SMA to acquire patient- specific induced motor neurons that were directly converted from fibroblasts via the forced expression of 8 defined transcription factors. The infected fibroblasts began to grow in a dipolar manner, and the nuclei gradually enlarged. Typical Tuj1-positive neurons were generated at day 23. After day 35, induced neurons with multiple neurites were observed, and these neurons also expressed the hallmarks of Tuj1, HB9, ISL1 and CHAT. The conversion efficiencies were approximately 5.8% and 5.5% in the SMA and control groups, respectively. -

Regulation of Adult Neurogenesis in Mammalian Brain
International Journal of Molecular Sciences Review Regulation of Adult Neurogenesis in Mammalian Brain 1,2, 3, 3,4 Maria Victoria Niklison-Chirou y, Massimiliano Agostini y, Ivano Amelio and Gerry Melino 3,* 1 Centre for Therapeutic Innovation (CTI-Bath), Department of Pharmacy & Pharmacology, University of Bath, Bath BA2 7AY, UK; [email protected] 2 Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK 3 Department of Experimental Medicine, TOR, University of Rome “Tor Vergata”, 00133 Rome, Italy; [email protected] (M.A.); [email protected] (I.A.) 4 School of Life Sciences, University of Nottingham, Nottingham NG7 2HU, UK * Correspondence: [email protected] These authors contributed equally to this work. y Received: 18 May 2020; Accepted: 7 July 2020; Published: 9 July 2020 Abstract: Adult neurogenesis is a multistage process by which neurons are generated and integrated into existing neuronal circuits. In the adult brain, neurogenesis is mainly localized in two specialized niches, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Neurogenesis plays a fundamental role in postnatal brain, where it is required for neuronal plasticity. Moreover, perturbation of adult neurogenesis contributes to several human diseases, including cognitive impairment and neurodegenerative diseases. The interplay between extrinsic and intrinsic factors is fundamental in regulating neurogenesis. Over the past decades, several studies on intrinsic pathways, including transcription factors, have highlighted their fundamental role in regulating every stage of neurogenesis. However, it is likely that transcriptional regulation is part of a more sophisticated regulatory network, which includes epigenetic modifications, non-coding RNAs and metabolic pathways.